Introduction
The increasing demand for plant-based proteins in the food industry has led to a growing interest in alternative protein sources, particularly those derived from underutilized food by-products. Sesame (Sesamum indicum L.) and perilla (Perilla frutescens L.) are widely cultivated oilseeds, and the residual meals generated during oil extraction processes are rich in proteins and bioactive compounds (Kim & Yoon, 2020; Saini et al., 2018). Despite their nutritional potential, these by-products are often discarded or relegated to low-value applications such as livestock feed (Saatchi et al., 2019). However, sesame and perilla meals represent sustainable, low-cost sources of plant proteins with favorable amino acid profiles, including essential amino acids (Jeon et al., 2016), making them promising candidates for incorporation into various food applications.
Protein functionality is highly dependent on intrinsic factors such as amino acid composition and molecular structure, as well as external factors, including pH, temperature, and ionic strength of the food matrix. From a production standpoint, the choice of extraction method is critical for maximizing protein functionality while ensuring environmental sustainability (Kumar et al., 2021). Conventional defatting methods often rely on organic solvents such as hexane, which are effective but raise environmental and health concerns due to residual solvent contamination and potential protein denaturation (L’hocine et al., 2006). Moreover, solvent-based extractions can alter protein structure, adversely affecting functional properties such as solubility and emulsification (Gravel et al., 2023; Kumar et al., 2021).
Soy protein isolate (SPI), a widely used plant-based protein in the food industry, is known for its excellent functional properties, including solubility, emulsification, and water and fat absorption capacities (Huang et al., 2023; L’hocine et al., 2006). Nevertheless, the environmental impacts associated with large-scale soybean cultivation, such as deforestation and biodiversity loss, have heightened the demand for alternative plant protein sources (Thrane et al., 2024). Recently, functional properties of proteins extracted from various plant-based sources—such as walnut, cottonseed, hempseed, sunflower, and chickpeas—have been studied as a means of recycling food by-products (Hadnađev et al., 2018; Ma et al., 2018; Malik et al., 2017; Malik & Saini, 2018; Mao et al., 2012). Studies on sesame and perilla meals, the by-products of sesame and perilla processing, have also been continuously reported (Kim & Yoon, 2020; Kim et al., 2023; Saini et al., 2018; Song et al., 2015), but research specifically focusing on their protein extracts remains limited.
Therefore, the present study aims to extract high-protein fractions from sesame and perilla meals using hot water extraction and to evaluate the functional properties of sesame meal protein extract (SMPE) and perilla meal protein extract (PMPE), focusing on their solubility, emulsifying properties, and absorption capacities. These properties were compared to those of SPI to assess the potential of SMPE and PMPE as viable alternatives in food formulations. This study is expected to provide information into the value-added utilization of oilseed by-products and contribute to the development of sustainable protein sources for the food industry.
Materials and Methods
Sesame meal and perilla meal pellets, the principal by-product resulting from oil processing, were provided by Queensbucket Co. Ltd. (Seoul, Korea). SPI with a protein content of 91.9% was obtained from Suihua Jinlong Oil Co. Ltd. (Suihua, China). Soybean oil was procured from Ottogi Co. Ltd. (Pyeongtaek, Korea). All other chemicals used were of analytical grade.
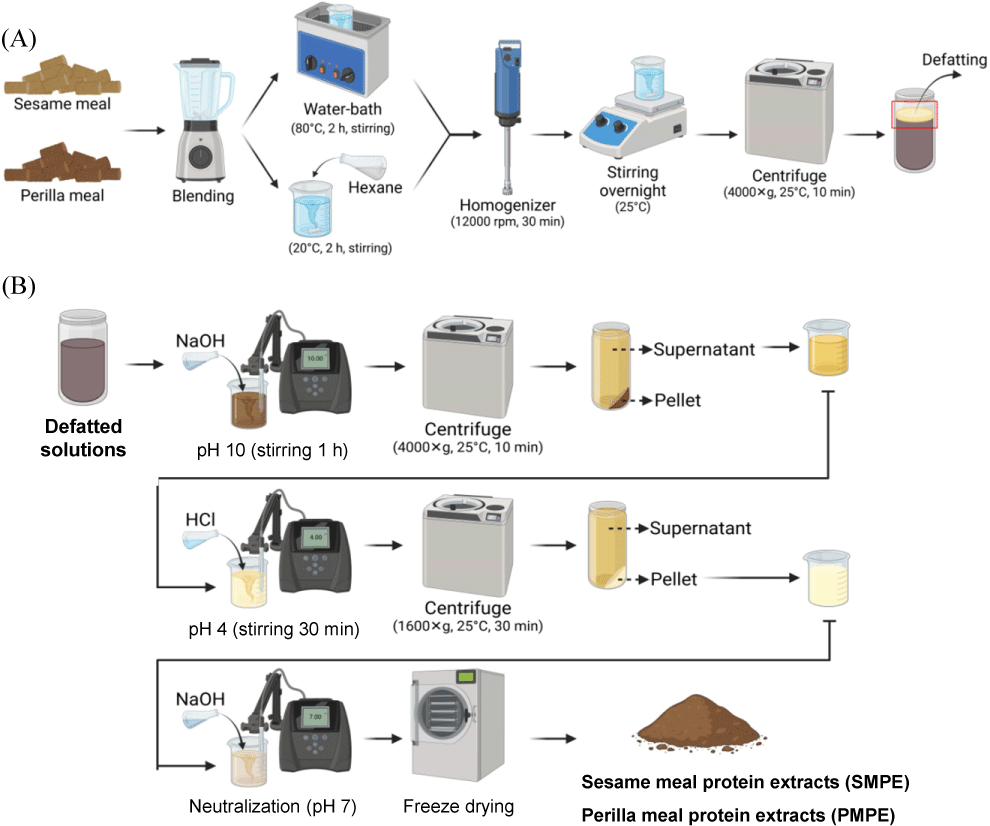
Sesame meal and perilla meal pellets were first pulverized using a laboratory grinder (Tepal, BL4258KR, Sarcelles, France), and then defatted using either the conventional hexane method or an environmentally friendly hot water method, respectively. For hexane defatting, 10 g of meal powder was mixed with n-hexane at a 1:10 (w/v) sesame-to-solvent ratio and stirred at 20°C for 2 h. For the hot water method, the meal powder was heated with 60 mL of distilled water at 80°C for 2 h while being continuously stirred. The resulting mixture was then homogenized for 30 min using a high-speed homogenizer (T25 digital ULTRA-TURRAX®, IKA, Staufen, Germany). After being stirred overnight at 25°C, the oil layer was removed by separating it with a centrifuge (Supra 22K, Hanil Science Industrial Co. Inchon, Korea) at 4,000×g for 10 min at 25°C.
The defatted solutions were adjusted to pH 10.0 using 1 M NaOH and stirred gently for 1 h. After centrifugation at 4,000×g for 10 min, the supernatant was acidified to pH 4.0 using 1 M HCl and stirred for another 30 min to induce precipitation. The precipitate was then isolated by centrifugation at 1,600×g for 30 min, resuspended in distilled water, and neutralized to pH 7 with 1 M NaOH. The resulting solutions were finally lyophilized to obtain SMPE and PMPE powders. A schematic diagram of protein extraction is shown in Fig. 1.
The protein and carbohydrate contents of SMPE and PMPE were analyzed following the AOAC official method (Schneeman, 1986). The amino acid composition of protein samples was analyzed using a high-performance liquid chromatography (HPLC) system (Ultimate 3000, Thermo Dionex, Waltham, MA, USA) equipped with a diode array detector (DAD-3000) and an Inno C18 column (4.6 mm×150 mm, 5 μm; Youngjin biochrom Co. Ltd. Seongnam, Korea). The mobile phase consisted of two components: mobile phase A (40 mM sodium phosphate, pH 7.0) and mobile phase B (water/acetonitrile/methanol, 10:45:45, by vol.). A gradient of mobile phase A was applied, decreasing from 95% to 20% over 30 min. The flow rate was maintained at 1.5 mL/min, with an injection volume of 0.5 μL. Data acquisition and chromatographic control were performed using Thermo Scientific™ Chromeleon™ 6.8 chromatography data system software.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 12% (w/v) separating gel and a 4% (w/v) stacking gel according to the previous method (Laemmli et al., 1970). A 10% (w/v) protein solution was dispersed in distilled water and boiled (at 95°C for 10 min) to induce protein denaturation. Electrophoresis was conducted using a Mini-PROTEAN® TGX™ Precast Gel (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with a Mini-PROTEAN electrophoresis system. The molecular weight range of the protein samples was determined using the Prosi Prestained Protein Marker (GenDEPOT, Barker, TX, USA).
Differential scanning calorimetry (DSC) was employed to evaluate the enthalpy changes related to thermal denaturation and heat treatment of proteins. The thermal properties of the proteins, including glass transition temperature (Tg), denaturation temperature (Td), and enthalpy of denaturation (ΔH), were determined using a differential scanning calorimeter (Perkin-Elmer DSC 4000, Norwalk, CT, USA). The DSC instrument was calibrated with indium metal. Protein samples (2.5 mg) and excess water (7.5 mg) were placed in an aluminum pan and tightly sealed. The DSC analysis was conducted under nitrogen at a heating rate of 10°C/min, ranging from 20°C to 200°C. ΔH values were expressed as a function of protein weight.
Protein solubility was analyzed following the method of Klompong et al. (2007). Sample solutions (2 mg/mL) were adjusted to pH values ranging from 2.0 to 10.0, stirred continuously for 1 h, and then centrifuged at 7,500×g for 15 min. The protein content in the supernatant was determined using the Bradford assay (1976). Solubility (%) was calculated as the ratio of the protein content in the supernatant (mg) to the total protein content of the sample (mg), expressed as a percentage.
The emulsifying properties were assessed based on the emulsifying activity index (EAI) and the emulsion stability index (ESI), with slight modifications to the method described by Klompong et al. (2007). A protein solution (30 mg/30 mL in distilled water) was mixed with 10 mL of soybean oil, and the pH was adjusted from 2.0 to 10.0. The mixture was then homogenized at 20,000 rpm for 1 min using a high-speed homogenizer. After allowing the emulsion to stand for 10 min, 50 μL aliquots were diluted with 5 mL of a 1 mg/mL SDS solution. Absorbance was recorded at 500 nm using a spectrophotometer (UV-1800, Shimadzu Co. Kyoto, Japan) immediately after preparation (A0) and again after 10 min of storage (A10). EAI and ESI were calculated according to the previous study by Kim et al. (2023).
Water absorption capacity (WAC) and fat absorption capacity (FAC) were measured according to the methods of Rodriguez-Ambriz et al. (2005) and Lin and Zayas (1987), respectively. A protein suspension (0.1 g/mL) in distilled water or soybean oil was vigorously vortexed for 30 s and then centrifuged at 12,879×g for 20 min. The supernatant was carefully decanted, and the tube was tilted at a 45° angle for 10 min to allow for complete drainage. WAC and FAC were calculated by dividing the volume of water or oil absorbed (mL) by the weight of the protein sample (g).
The experimental data was collected in triplicate, and the results are presented as means±standard deviations (SD). To evaluate the variance between means, a one-way ANOVA was performed, followed by Tukey’s HSD multiple range test to identify significant differences (p<0.05) among the datasets. Statistical analyses were carried out using IBM SPSS Statistics 26 (IBM SPSS Statistics, Chicago, IL, USA).
Results and Discussion
The protein contents of SMPE and PMPE were analyzed following hexane defatting and hot water defatting using the Bradford (1976) method, with no significant differences observed between the two defatting techniques (Fig. S1). Given the comparable efficiencies and the environmental benefits, hot water defatting was selected for further protein extraction. The extraction yields of SMPI and PMPE were 20.21±0.87% and 20.57±0.60%, respectively. The protein contents of the final extracted SMPE and PMPE, determined using the AOAC method, are presented in Table 1. The protein contents of SMPE and PMPE, extracted from sesame meal (containing 37.33% protein) and perilla meal (containing 37.16% protein), respectively, were significantly different. SMPE exhibited a protein content of 50.74%, while PMPE had a higher protein content of 66.83%. These values were similar to or lower than previously reported protein contents for sesame protein concentrates or isolates, which ranged from 54.05%–55.43% (Yang et al., 2021), 78.3% (Chatterjee et al., 2025), and up to 88.05% (Mathews et al., 2022). Likewise, the protein content of PMPE was comparable to values reported for perilla protein extracts, which ranged from 67.87% to 73.48% (Zhao et al., 2021). The carbohydrate content of PMPE (12.64%) was considerably higher than that of SMPE (4.74%). This difference may result from compositional variations between sesame and perilla meals, with perilla meals likely to contain a higher amount of fiber or other polysaccharides.
The amino acid profiles of SMPE and PMPE showed distinct compositional differences (Table 1 and Fig. S2). SMPE contained significantly higher levels of hydrophobic amino acids (1,951.25 mg/kg) compared to PMPE (797.26 mg/kg). These hydrophobic amino acids, which are essential for protein structural stability and interfacial activity, suggest that SMPE may be more suitable for applications requiring enhanced hydrophobic interactions (Kim et al., 2023; Ghanbarinia et al., 2022). Notably, leucine was highly abundant in SMPE (518.03 mg/kg), potentially enhancing both its nutritional value and functional roles, particularly in protein synthesis and metabolic regulation (Duan et al., 2016). Conversely, PMPE was substantially richer in hydrophilic amino acids (817.51 mg/kg) than SMPE (450.44 mg/kg), suggesting greater compatibility with aqueous-based food systems, such as beverages. In particular, PMPE exhibited elevated concentrations of glutamic acid and glutamine, which are important in metabolic pathways and contribute to umami flavor (Brosnan & Brosnan, 2013).
Overall, alanine, phenylalanine, and valine were more abundant in SMPE, potentially contributing to its higher protein structural complexity. Conversely, glutamine, glutamic acid, and arginine were more abundant in PMPE, indicating that it may have different metabolic functions or health benefits, particularly in supporting digestive health and immune function (Achouri et al., 2012; Ren et al., 2013).
The SDS-PAGE analysis of SMPE and PMPE extracted from sesame and perilla meals revealed protein bands within the molecular weight range of 5 to 55 kDa (Fig. 2), suggesting the presence of a diverse array of protein subunits. The predominant storage proteins identified in both extracts were 11S globulin (α-globulin) and 2S albumin (β-globulin), both of which play critical roles in seed storage and contribute to protein functionality (Orruno & Morgan, 2007). The 11S globulin in SMPE and PMPE was primarily composed of two major subunits: an acidic subunit (30–35 kDa) and a basic subunit (20–25 kDa), consistent with the polypeptide distribution reported for globulin proteins in other plant-based protein sources (Saatchi et al., 2019). These subunits contribute to the structural integrity and functional properties of the protein.

In addition, both extracts exhibited 2S albumin bands in the 4–9 kDa range, stabilized by disulfide bonds, in agreement with previous reports on the 2S albumin family (Achouri et al., 2012). 2S albumins typically function in seed defense and can also contribute to the solubility of protein, making them important for various food and industrial applications. Furthermore, 7S globulin was detected in both SMPE and PMPE, with a molecular weight of approx. 50 kDa. However, the bands corresponding to 7S globulin in SMPE were relatively weaker compared to PMPE. Overall, the results suggest that while both SMPE and PMPE share similar storage proteins, the variation in 7S globulin content and the relative intensities of different protein bands may contribute to differences in their functional properties, solubility, and industrial applications.
DSC was used to characterize the enthalpy changes associated with thermal denaturation and heat-induced transitions of proteins (Table 2). Protein denaturation occurs irreversibly upon initial heating, whereas the glass transition is reversible and can be detected in subsequent heating or cooling scans after the onset of a disordered arrangement (Ricci et al., 2018). The DSC profiles were compared with SPI (protein content: 91.9%), a widely used plant-based protein in the food industry. The glass transition temperature (Tg), which indicates the mobility of the amorphous regions within the protein matrix, did not differ significantly among samples (Table 2). The denaturation temperature (Td), representing the temperature at which protein denaturation occurs, was higher for SPI, indicating greater thermal stability. The higher Td in SPI could be attributed to a more ordered protein structure or stronger intermolecular interactions. Previous studies have reported that proteins with a higher content of nonpolar residues exhibit increased thermal stability, as nonpolar interactions contribute to the stabilization of protein tertiary structure (Alarape et al., 2024; Saini et al., 2018).
| Protein extracts1) | Tg (°C)2) | Td (°C) | ΔH (J/g) |
|---|---|---|---|
| SPI | 59.42±0.54NS,3) | 147.94±0.24a | 0.64±0.04a |
| SMPE | 58.02±0.57 | 101.10±4.26b | 1.43±0.06b |
| PMPE | 58.70±0.83 | 100.09±0.05b | 2.08±0.23c |
1) SPI, soy protein isolate; SMPE, sesame meal protein extracts; PMPE, perilla meal protein extracts
The enthalpy change of denaturation (ΔH) reflects the energy required to disrupt the protein’s native structure, providing information into hydrophobic/ hydrophilic interactions and protein compactness (Ma & Harwelkar, 1991). In this study, SPI exhibited relatively lower ΔH values than SMPE and PMPE, suggesting a less compact structure with reduced secondary structural elements, such as α-helices, and a greater proportion of random coil formations (Koshiyama et al., 1981). These findings suggest that while SPI exhibits higher thermal stability (higher Td), SMPE and PMPE possess tighter molecular packing (higher ΔH), potentially affecting their performance in thermally processed food systems.
Fig. 3 illustrates the solubility profiles of SPI, SMPE, and PMPE across different pH levels. All protein extracts exhibited the lowest solubility at pH 4, consistent with previous findings indicating that protein extracts typically display a U-shaped solubility curve, with the minimum solubility occurring between pH 4 and 5 (Khalid et al., 2003; Sahni et al., 2020). This behavior is closely associated with the isoelectric point (pI), where the net charge of the protein is near zero, leading to reduced electrostatic repulsion and increased protein aggregation (Liu et al., 2018). Similar trends have been observed in other plant protein isolates, where solubility is influenced by pH-dependent conformational changes and charge distribution (Sridhar & Bhat, 2007; Li et al., 2020).
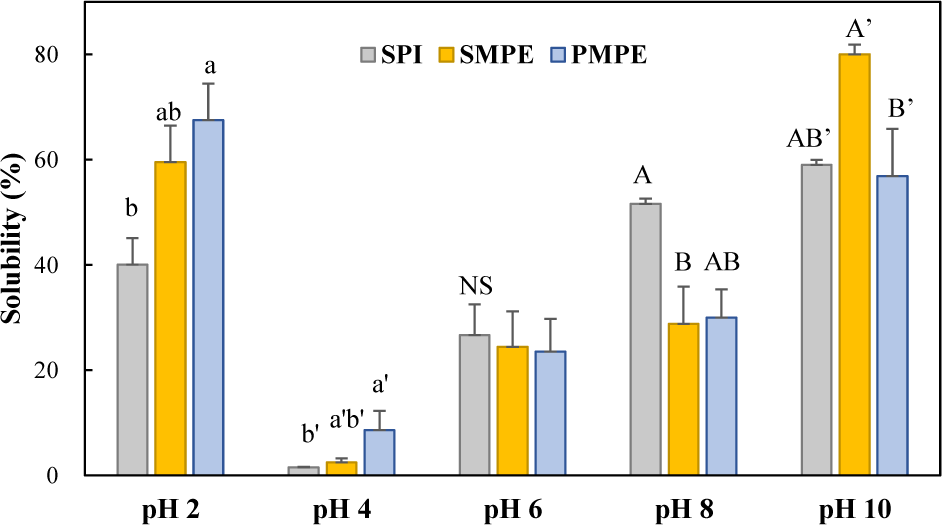
The studied protein extracts demonstrated good solubility in both acidic and alkaline pH ranges, which are a crucial characteristic for food applications (Rashwan et al., 2025). Beyond the isoelectric point, solubility increased with rising pH, demonstrating a typical protein solubility pattern. At higher pH levels, proteins acquire a greater net negative charge, enhancing electrostatic repulsion and promoting interactions with water molecules, thereby improving solubility. Notably, SMPE demonstrated significantly higher solubility at pH 10 compared to both SPI and PMPE. This enhanced solubility may be attributed to variations in amino acid composition, particularly its higher hydrophobic residue content, or structural differences that confer improved water-binding and dispersion capabilities under alkaline conditions.
EAI, which reflects the ability of proteins to adsorb at the oil-water interface, is presented in Fig. 4A. Across various pH levels, all protein extracts exhibited the lowest EAI at pH 4, aligning with the solubility results. At this pH, which corresponds to the pI, proteins are less soluble and exhibit reduced electrostatic repulsion. Due to their low solubility at the pI, protein molecules experience strong intermolecular interactions, preventing their efficient migration to the oil-water interface (Mao & Hua, 2012). As the pH deviates from the isoelectric point, EAI values increased significantly for all protein extracts. This trend may be attributed to partial unfolding or denaturation of proteins, which enhances adsorption and rearrangement at the oil-water interface (Sahni et al., 2018). Given that emulsifying ability is influenced by the hydrophilic-lipophilic balance, which varies with pH (Zhao et al., 2022), the observed pH dependence of EAI was anticipated. Notably, SMPE demonstrated a greater increase in emulsifying ability under alkaline conditions than in acidic ones. Emulsifying properties are strongly affected by ionic charge and surface hydrophobicity. At higher pH levels, protein unfolding and hydrolysis can expose hydrophobic groups, reduce the surface charge on oil droplets, and enhance emulsifying efficiency in alkaline environments (Zhao et al., 2022).

The ESI, which measures the ability of proteins to maintain emulsion stability over time, is shown in Fig. 4B. Overall, SMPE exhibited significantly higher ESI than PMPE across all pH levels but did not surpass SPI. Below the isoelectric point, both SMPE and PMPE showed relatively higher ESI values, while a notable increase in SPI stability was observed at pH 6 and 8. This suggests that, in addition to solubility, other physicochemical factors such as particle size and zeta potential may play a role in emulsion stability (Wang et al., 2021).
WAC and fat absorption capacity FAC are essential indicators for assessing the emulsifying properties of proteins, as they reflect the ability of proteins to retain water and fat (Ren et al., 2019). These properties are crucial for applications in food systems, particularly in formulations requiring emulsification, such as in the production of meat analogs, dressings, and spreads. WAC indicates the hydrophilicity of a protein, whereas FAC reflects its lipophilicity—both of which influence the emulsifying capacity and stability of proteins at the oil-water interface. The WAC values, shown in Fig. 5 demonstrated the highest value for SPI. This is consistent with previous studies, which suggest that high solubility in water often correlates with greater water retention due to the formation of a protein-water matrix (Sathe, 2012). The network structure facilitates water entrapment by promoting the interaction between water molecules and the hydrophilic regions of proteins, such as polar amino acids or charged side chains. This higher WAC in SPI may be related to its greater number of hydrophilic amino acid residues, which are known to enhance water binding (Ma et al., 2022).

On the other hand, FAC was significantly higher in SMPE compared to SPI and PMPE. The higher FAC value of SMPE can be attributed to its greater surface hydrophobicity, resulting from a higher proportion of hydrophobic or nonpolar amino acids —a trend observed in other plant proteins with higher hydrophobic content (Malik & Saini, 2018). This characteristic allows SMPE to interact more effectively with oil molecules and enhances its ability to absorb fat. In the case of SPI, which showed the lowest FAC value, likely due to its relatively lower hydrophobicity and greater tendency to form a highly soluble, water-bound structure, which reduces its ability to interact with oils (Kakar et al., 2022). Further investigations into the molecular structure of these proteins and their interaction with different solvents are needed to better understand the underlying mechanisms governing WAC and FAC. The current study aligns with findings by Mao et al. (2012), who observed a similar trend in protein isolates derived from walnuts, where proteins with higher hydrophobic amino acid content exhibited enhanced fat absorption properties.
Conclusion
This study evaluated the functional properties of SMPE and PMPE, obtained through hot water defatting and acid precipitation, and compared them to SPI. SMPE and PMPE exhibited protein contents of 50.74% and 66.83%, respectively, with SMPE having a higher concentration of hydrophobic amino acids and a relatively lower proportion of 7S globulin compared to PMPE. The solubility of all protein extracts was lowest at pH 4, with a significant increase observed in SMPE at pH 10. The emulsifying properties of both SMPE and PMPE were pH-dependent, with SMPE exhibiting superior emulsifying activity and stability at alkaline pH compared to SPI and PMPE. Additionally, the study suggested that SMPE, with its higher FAC, could be more effective in applications requiring fat-binding abilities, while SPI, with a higher WAC, demonstrated a better ability to retain water. Overall, the results indicate that SMPE and PMPE, as plant-based protein extracts from food by-products, possess promising functional properties that could be valuable in various food applications, particularly those requiring emulsification, fat absorption, and water retention. SMPE, due to its high fat absorption and emulsifying capacity, may be applicable to plant-based meat substitutes and emulsified sauces such as salad dressings and mayonnaise alternatives. PMPE, with its higher hydrophilic amino acid content and water solubility, may be better suited for use in dairy substitutes such as plant-based milk, yogurt, and protein-enriched beverages. These findings support the potential of SMPE and PMPE as sustainable alternatives to SPI, contributing to the added-value utilization of plant-based food by-products. Further research is needed to investigate the molecular mechanisms behind these functional differences and to optimize their application in diverse food systems.







