Introduction
The growing global population has driven a continuous surge in protein demand, prompting the search for sustainable and affordable alternatives to animal-derived proteins. Plant-based proteins have emerged as attractive candidates due to their nutritional benefits, lower production costs, and renewable nature. One promising avenue is the recovery of proteins from agricultural by-products, which can simultaneously reduce environmental waste and create value-added products (Zhou et al., 2023). Sesame, widely recognized for its health-promoting oil rich in linoleic acid, generates a nutrient-dense residue as a by-product of oil extraction. This sesame residue, containing about 28–45% protein, is not only flavorful but also possesses favorable functional properties for food applications (Onsaard, 2012). However, despite its potential, sesame residue is often underutilized, commonly diverted to low-value uses such as animal feed, fuel, or fertilizer, and in some cases, discarded—resulting in resource inefficiency and environmental challenges (Onsaard, 2012; Kim et al., 2023). Unlocking the functional potential of sesame protein isolates from these residues could significantly improve the economic and environmental sustainability of sesame processing, paving the way for their incorporation into diverse food systems.
Proteins in food systems possess both hydrophilic charged regions and hydrophobic domains, enabling them to reduce surface tension and stabilize emulsion interfaces. However, their functional and emulsifying capacities are often constrained by challenges such as poor solubility, structural instability, pH sensitivity, and limited interfacial activity (Qu et al., 2018). One effective strategy to overcome these limitations is protein conjugation with polysaccharides via the Maillard reaction, a non-enzymatic process in which reducing sugars covalently bind to free amino groups of proteins or peptides (Ali et al., 2019; Saatchi et al., 2019). This reaction has been widely recognized for enhancing the functional attributes of proteins, including solubility, thermal stability, and emulsifying performance. Among various protein modification techniques—physical, chemical, and enzymatic—the Maillard reaction offers a particularly advantageous and sustainable route to improve protein functionality (de Oliveira et al., 2016). Recent research has explored the impact of protein–polysaccharide conjugation on the structural and functional properties of various plant-based protein isolates, such as those from canola, oat, rice, and soy (Higa et al., 2023).
Based on this, the present study focuses on the dry heat-induced Maillard conjugation of sesame meal protein extract (SMPE) with maltodextrin (MD) of varying dextrose equivalents (DE). SMPE was obtained from sesame meal, a valuable food processing by-product, through hot water defatting and acid precipitation. This work aimed to elucidate the structural changes and functional enhancements induced by MD conjugation, providing information into the potential application of SMPE-MD conjugates as functional ingredients in the food industry.
Materials and Methods
Sesame meal pellet, a food by-product, was obtained from Queensbucket Co. Ltd. (Seoul, Korea) and ground using a laboratory grinder. MD with a DE of 8–10 was supplied by Samyang Genex Co. (Seoul, Korea), and MD with DE ranges of 4–7 and 16.5–19.5 was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Soybean oil was purchased from a local market (Ottogi Co. Ltd. Pyeongtaek, Korea). All other chemicals used were of analytical grade.
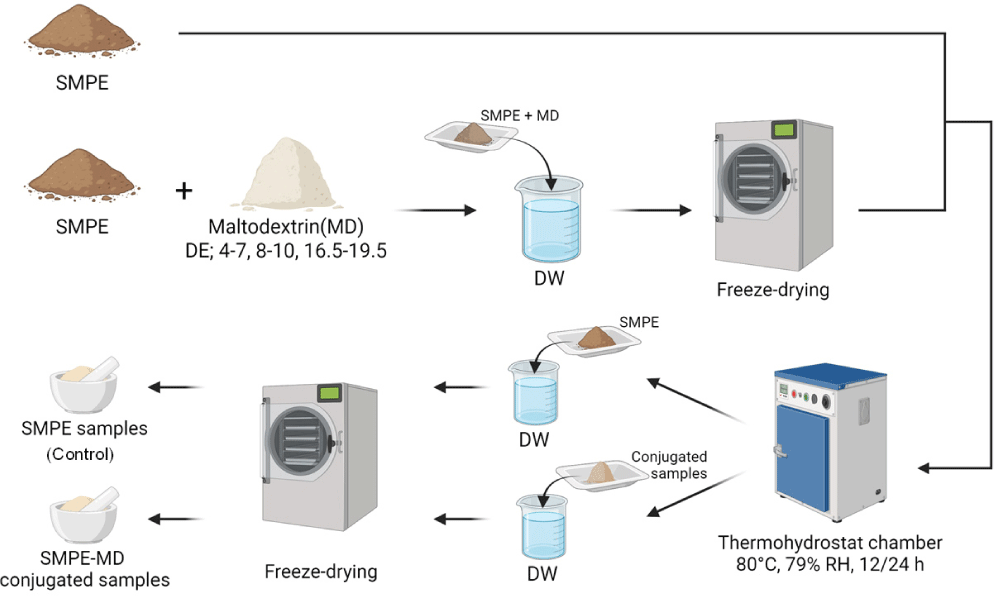
SMPE (protein content 50.74%) was extracted from sesame meal pellets by sequential hot water defatting and precipitation processes (Kim et al., 2023). The SMPE-MD conjugates were prepared via the dry-heating Maillard reaction as described by Kato et al. (1990), and the process is illustrated in Fig. 1. A mixture of SMPE (6 g) and MD (DE 4–7, 8–10, and 16.5–19.5) at a weight ratio of 2:1 (w/w) was dissolved in 500 mL distilled water and subsequently freeze-dried using a lyophilizer (FD8508, Ilshin BioBase Co. Ltd., Dongducheon, Korea). The resulting protein– carbohydrate powder was subjected to dry-heating in a thermohygrostat chamber (PTHC-81R, lab house Co. Ltd., Pocheon, Korea) maintained at 80°C and 79% relative humidity for 12 and 24 h to promote the Maillard reaction. The conjugated samples were rehydrated in distilled water, followed by lyophilization, and subsequently ground into fine powder using a mortar to obtain the final powdered conjugates (SMPE-MD conjugates). SMPE heated under the same conditions without MD was used as a control.
Protein molecular weight distribution was analyzed by SDS-PAGE following the method outlined by Laemmli (1970). Protein samples (0.1 g/mL) were dissolved in distilled water and subjected to heat treatment at 95°C for 10 min to facilitate denaturation. The denatured proteins were then loaded onto the wells of Mini-PROTEAN® TGX™ Precast Gel (Bio-Rad Laboratories, Hercules, CA, USA), consisting of 12% separation gel and 4% stacking gel. Protein band separation was performed using a Mini-PROTEAN electrophoresis system at a constant voltage of 160 V. Protein molecular weight distribution was estimated using Prosi Prestained Protein Marker (GenDEPOT, Barker, TX, USA) as a molecular weight standard.
FT-IR spectra of the samples were obtained using an FT-IR spectrometer (Vertex 80v, Bruker Optics GmbH, Ettlingen, Germany) equipped with a deuterated triglycine sulphate (DTGS) detector. Data acquisition was performed using Opus 7.8 software. The samples were ground and mixed with potassium bromide (KBr), then pressed into pellets. Spectra were obtained in the wavenumber range of 600–4,000 cm–1 with 16 scans at a resolution of 4 cm–1.
The solubility of SMPE-MD conjugates was determined using the Bradford method (Bradford, 1976). The lyophilized samples were dispersed in distilled water to prepare a concentration of 2 mg/mL and adjusted to pH ranging from 2.0 to 10.0 using 1 N HCl and NaOH. Each solution was stirred for 1 h and subsequently centrifuged at 7,500×g for 15 min using centrifuge (Supra 22K, Hanil Science Industrial Co., Incheon, Korea). A 20 μL aliquot of the supernatant was mixed with 1 mL of Bradford reagent and incubated for 5 min, after which the absorbance was measured at 595 nm using a UV-Vis spectrophotometer (UV-1800, Shimadzu Co., Kyoto, Japan). The protein concentration was determined based on a standard curve generated using bovine serum albumin (BSA) as the reference protein. The solubility of the SMPE-MD conjugates was calculated using the following Eq. (1):
Emulsifying activity index (EAI) and emulsion stability index (ESI) were evaluated using a turbidimetric method based on the procedure of Klompong et al. (2007) with slight modifications. To prepare the emulsion, 30 mL of SME-MD conjugate solution (1 mg/mL) was mixed with 10 mL of soybean oil, adjusted to pH values ranging from 2 to 10, and homogenized at 20,000 rpm for 1 min using a high-speed homogenizer (T25 digital ULTRA-TURRAX®, IKA, Staufen, Germany). After standing for 10 min, 50 μL of the emulsion was diluted with 5 mL of a 1 mg/mL SDS solution. The absorbance of the diluted emulsion was measured at 500 nm using a UV-Vis spectrophotometer. EAI and ESI were calculated by using Eqs. (2) and (3), respectively:
where A0 and A10 represent the absorbance measured immediately (0 min) after homogenization and after 10 min of storage following homogenization, respectively. Δt denotes the time interval between these two measurements.
All measurements were performed in triplicate, and the results are expressed as mean values ± standard deviations (SD). One-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test, was used to assess differences among sample means. Additionally, three-way ANOVA was conducted to evaluate the effects and interactions of the independent variables (MD type, dry-heating time, and pH) on the dependent variables. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA), and differences were considered statistically significant at p<0.05.
Results and Discussion
In this study, MD was selected as the glycosyl donor for Maillard-type conjugation with SMPE due to its non-toxic nature, affordability, and broad applications in food and pharmaceutical industries. The Maillard reaction, a widely employed non-enzymatic browning process, enhances protein functionality by covalently linking reducing sugars to free amino groups in proteins or peptides (de Oliveira et al., 2016). This reaction progresses through three distinct phases: the early stage, involving the formation of a reversible Schiff base and subsequent Amadori rearrangement; the intermediate stage, marked by sugar degradation and amino acid breakdown; and the advanced stage, where complex reactions yield nitrogenous heterocycles and melanoidins (de Oliveira et al., 2016). While early-stage Maillard products are generally colorless and exhibit improved techno-functional properties such as emulsification, thermal stability, and foaming, later stages are associated with color development and bioactive compounds with antioxidant and antimicrobial properties. Key factors influencing the efficiency and safety of Maillard conjugation include the molecular characteristics of the reactants, the protein-to-carbohydrate molar ratio, availability of reactive functional groups, pH, water activity, temperature, and heating duration, all of which determine the functionality of the final conjugates (Zha et al., 2021; Naik et al., 2022).
When the dried mixture of SMPE and MD was subjected to heat treatment at 80°C, it exhibited a light brown appearance (data not shown), suggesting Maillard reactions or other structural modifications. The SDS-PAGE analysis revealed that SMPE initially displayed characteristic bands (2s albumins, 11s globulins, and 7s globulins) in the molecular weight range of 5–55 kDa, which changed after conjugation with MD (Fig. 2). As the SMPE-MD conjugation reaction progressed, the intensity of the characteristic protein bands of SMPE gradually decreased. The 7S globulin band at approx. 56 kDa gradually faded, and a new band emerged around 170 kDa, suggesting the formation of a high-molecular-weight conjugate. This observation is consistent with previous studies suggesting that it may result from molecular modifications within the protein itself or from its involvement in Maillard reaction with polysaccharides (de Oliveira et al., 20l6; Zha et al., 2021).

Additionally, the disappearance of the 30–35 kDa band suggests the involvement of the 11S globulin acidic subunit in the conjugation. Previous studies have reported that the 11S globulin subunit contains reactive lysine residues that can readily participate in glycation reactions (Hiller & Lorenzen., 2010; Zhang et al., 2014). As heat treatment time increased, the extent of glycosylation intensified, leading to further reduction in free amino groups. This phenomenon occurs due to the reaction (covalent bond) between the free amino groups of proteins and the reducing carbonyl groups of polysaccharides, which progressively decreases the number of reactive amino groups, indicating the progression of the Maillard reaction (Hiller & Lorenzen, 2010; de Oliver et al., 2016).
To investigate the structural interactions between proteins and polysaccharides in the conjugated system, the FT-IR spectra of SMPE-MD conjugates were analyzed (Fig. 3). The absorption peaks corresponding to chemical bonds involved in amide group formation were observed in the mid-infrared spectrum, which is closely linked to Maillard reaction products (Van Der Ven et al., 2002). Fig. 3 presents the structural changes of SMPE and SMPE-MD conjugates in the dry powder state. In MD, a characteristic band at 2,900 cm–1 was attributed to C–H stretching. A broad band at 3,318 cm–1 was assigned to hydrogen-bonded hydroxyl groups, indicating the presence of free, intermolecular, and intramolecularly bound hydroxyl groups, which constitute the polysaccharide’s overall structure (Zhang et al., 2018). Additionally, characteristic bands at 924 and 1,149 cm–1 were associated with C–O stretching of the anhydrous glucose ring (C–O–C bond) and C–O stretching of hydroxyl groups (C–O–H), respectively. In the protein spectrum, SMPE was observed to have distinct peaks associated with amide functional groups; amide A (free and bound O–H and N–H stretching) and amide B (C–H stretching) were detected at 3,000–3,500 cm–1 and 2,850–2,980 cm–1, respectively (Ren et al., 2019). The amide I band at 1,700–1,600 cm–1 (C=O stretching) represents secondary structural components, such as α-helices, β-sheets, β-turns, β-antiparallel structures, and random coils (Wang et al., 2014; Mir et al., 2020). Furthermore, amide II (N–H deformation) and amide III (C–N stretching and N–H deformation) bands were found at 1,600–1,500 cm–1 and 1,300–1,200 cm–1, respectively (Demirkiran et al., 2022).

Upon conjugation with MD via dry heating, notable changes were observed in the FTIR spectra. A slight redshift of the O–H stretching vibration from 3,318 cm–1 to 3,282 cm–1 was detected in the SMPE-MD conjugates, indicating enhanced hydrogen bonding between the two biopolymers (Nooshkam & Madadlou, 2016; Pirestani et al., 2018). Additionally, an increase in absorption intensity in the amide and carbohydrate-associated regions suggested successful glycosylation. The enhancement of absorption peaks in the C–O stretching (1,149 cm–1) and hydroxyl group (3,318 cm–1) regions was attributed to the increased presence of C–O bonds and hydroxyl groups, a hallmark of protein-polysaccharide covalent conjugates (Pirestani et al., 2018). Furthermore, shifts and decreased intensities in the amide I, II, and III bands to 1,627, 1,540, and 1,242 cm–1, respectively, were observed in the SMPE-MD conjugates. These band shifts and intensity decreases suggest structural modifications associated with Maillard-driven covalent bonding, particularly involving lysine residues (Wang et al., 2013; Saatchi et al., 2019). Given that the Maillard reaction predominantly involves nucleophilic attack by free amino groups (especially lysine ε-amino groups) on reducing sugars, the observed changes indicate a potential loss of free lysine functional groups due to glycation (Huang et al., 2012; Li et al., 2021). Such modifications are consistent with previous studies reporting altered secondary protein structures, reduced free amino groups, and increased molecular weight following glycosylation (Zhang et al., 2014).
The solubility changes of SMPE-MD conjugates are shown in Fig. 4. SMPE, which was heat-treated for the same duration (12 and 24 h) as the conjugates without MD, showed a slight increase in solubility when heated with MD. This improvement in solubility is likely attributed to the increased hydrophilicity introduced by the presence of MD. The extent of this enhancement was inversely correlated with the DE of MD, meaning that MD with a higher molecular weight (lower DE values) contributed more significantly to the solubility of SMPE-MD conjugates. Specifically, conjugates formed with MD of DE 4–7 exhibited the most pronounced increase in solubility over both 12 h and 24 h heat treatment durations. These findings suggest that the molecular size of polysaccharides plays a crucial role in stabilizing protein-polysaccharide interactions, with larger MD molecules facilitating stronger conjugation with SMPE and consequently improving solubility (Kutzli et al., 2020). In contrast, glycosylation involving MD with DE 8–10 and DE 16.5–19.5 resulted in higher solubility than heat-treated SMPE alone, but the increase was not statistically significant. This suggests that while covalent bonding between SMPE and MD can enhance the protein’s interaction with water molecules, the effectiveness of glycosylation varies depending on the MD molecular weight. Glycosylation is known to reduce protein aggregation, increase protein-solvent interactions, and decrease protein-protein interactions, all of which contribute to improved solubility (Zhang et al., 2018; Naeini et al., 2024).

The duration of heat treatment also significantly influenced solubility trends. For SMPE-MD DE 4–7 conjugates, solubility was higher when the conjugates were heated for 24 h compared to 12 h. However, for SMPE-MD DE 8–10 and DE 16.5–19.5 conjugates, solubility was higher at 12 h than at 24 h. Extended heating times can enhance glycation, leading to improved solubility up to a certain point. However, excessive heating may result in over-glycation, causing protein cross-linking and aggregation, which ultimately reduces solubility (Mulcahy et al., 2016). Similar observations have been reported for whey protein isolate (WPI) conjugated with gum acacia, where solubility initially improved due to glycation but declined upon excessive Maillard reaction progression beyond 24 h (Chen et al., 2019). These results indicate the importance of optimizing both MD molecular weight and reaction time to enhance the solubility of SMPE-MD conjugates.
The EAI and ESI of SMPE-MD conjugates were evaluated after heat treatment at 80°C for 12 and 24 h. At pH 4, which is near the isoelectric point of SMPE, all samples exhibited low EAI values, likely due to protein aggregation and reduced surface activity (Table 1 and Fig. 5). However, as the pH increased, the EAI values of all samples increased, and SMPE-MD conjugates exhibited significantly higher EAI values than SMPE alone, regardless of dry-heating time (Fig. 5A). This suggests that glycosylation through the Maillard reaction enhances emulsifying activity by suppressing protein aggregation via steric hindrance and improved solubility. These findings align with the study by Lee et al. (2004), which reported that MD contributes to steric stabilization by forming a protective layer around emulsifying droplets, thereby enhancing emulsion formation and stability. Additionally, conjugation with MD expands the secondary protein structure, exposing hydrophilic hydroxyl groups that facilitate adsorption at the oil-water interface, as suggested by Saatchi et al. (2019). Interestingly, the emulsifying activity was also influenced by the molecular size of the conjugates (Fig. 5A). Conjugates with MD of smaller molecular weight (DE 16.5–19.5) exhibited higher EAI values, which was consistent with the findings of Du et al. (2021), who demonstrated that proteins with smaller particle sizes showed greater emulsifying ability and stability due to their enhanced interfacial interactions.

The ESI values exhibited an inverse relationship with the EAI values across different pH levels, with a notably higher ESI observed at pH 4 (Fig. 5B). This could be attributed to the very low EAI at pH 4, likely resulting from a limited number of initially emulsified droplets. Consequently, although the turbidity ratio appeared lower at this pH, the absolute turbidity after 10 min was actually higher at elevated pH levels. Three-way ANOVA showed that all factors—pH, MD, and dry-heating time—significantly affected the ESI values (p<0.001). In particular, the conjugates prepared with smaller molecular weight MD (DE 16.5–19.5) showed significantly higher emulsifying stability than the control SMPE (p<0.001). Therefore, the results of this study indicate that the conjugated sample using MD with a lower molecular weight at pH 6 and dry-heating for 12 h was a favorable condition for improving the EAI and ESI of SMPE.
Conclusion
In this study, SMPE, a protein source extracted from food processing by-products, was used to investigate its structural and functional properties following conjugation with MD, thereby confirming its potential application in food systems. SMPE-MD conjugates were prepared via dry heat-induced glycosylation using MD with varying dextrose equivalents (DE 4–7, 8–10, and 16.5–19.5). Alterations observed in SDS-PAGE and FT-IR profiles confirmed that the conjugation of SMPE and MD occurred through the Maillard reaction. The solubility and emulsifying properties (EAI and ESI) of these conjugates were significantly influenced by both the DE of MD and the dry-heat treatment time. Solubility decreased with the increasing DE of MD but reached its peak after 12 h of dry-heating. In particular, SMPE-MD conjugates prepared with lower molecular weight MD (DE 16.5–19.5) showed significantly higher emulsifying stability compared to the control SMPE. These findings suggest that SMPE-MD conjugates have promising potential for functional food applications. Given their enhanced emulsifying stability, these conjugates may be particularly applicable in industrial formulations such as salad dressings, emulsified beverages, sauces, and plant-based dairy alternatives. However, the limited solubility of SMPE may hinder glycation efficiency by reducing the extent of molecular interaction and dispersion between protein and carbohydrate components. While this study employed prolonged stirring before freeze-drying to facilitate uniform hydration, future research should consider pretreatment strategies—such as pH adjustment, ultrasonication, high-pressure homogenization, or microwave processing—to enhance SMPE solubility and, consequently, bonding efficiency. These emerging technologies offer the potential to shorten reaction times, improve product uniformity, and reduce thermal damage during conjugation. Future studies focusing on the detailed optimization of processing conditions and further structural modifications may enhance the functionality of these conjugates and promote the value-added utilization of food processing by-products in various industrial formulations.







