Introduction
Agricultural products and their fermented derivatives have long been integral to human diets and are increasingly recognized as a rich source of functional ingredients that promote human health (Voidarou et al., 2020). In particular, agricultural products are not only rich in various dietary fibers, vitamins, minerals and phytochemicals, but also produce secondary metabolites such as organic acids, enzymes and bioactive peptides during fermentation. Such compounds are known to exhibit a broad spectrum of physiological activities including immune modulation, antioxidant and anti-inflammatory effects, as well as the prevention of metabolic disorders (Wallace et al., 2020; Fabbri et al., 2024). Nevertheless, systematic and integrative investigations into the functional properties of fermented agricultural products remain limited, and the potential of region-specific crops and traditional fermentation resources has yet to be fully explored. South Korea, with its distinct four seasons and diverse climatic and soil conditions, is particularly rich in agricultural resources. Harnessing these resources for the development of tailored functional foods and bioactive ingredients is regarded as a promising avenue with high potential to generate substantial added value both domestically and globally (Jung et al., 2022; Kim et al., 2024).
Green garlic (Allium sativum L.) is a monocotyledonous vegetable of the genus Allium (family Amaryllidaceae, order Asparagales). It is cultivated worldwide, with numerous region-specific cultivars reported (Sönmez et al., 2025). In South Korea, green garlic is primarily grown in the mild climates of southern coastal and inland regions and is particularly renowned as a local specialty in areas such as Sacheon (Gyeongnam) and Goheung (Jeonnam), due to its abundance of bioactive compounds (Yang et al., 2005, 2025). Morphologically, green garlic varies in size and form depending on harvest time but generally consists of slender, tender green leaf sheaths measuring 30–50 cm in length and 1–2 cm in diameter (Sönmez et al., 2025). Traditional Korean medical texts, including the Dongui Bogam, describe green garlic as effective in dispelling internal cold and strengthening the immune system. In modern studies, various physiological activities such as antioxidant, anti-inflammatory and antiviral effects have been scientifically validated (Bozin et al., 2008). Furthermore, green garlic is particularly rich in allicin, γ-glutamyl cysteine compounds, and vitamin C, which contribute to immune enhancement, regulation of blood cholesterol levels, and prevention of metabolic disorders (Skoczylas et al., 2023).
Recent studies employing lactic acid bacteria (LAB) and Bacillus strains granted Qualified Presumption of Safety (QPS) status by the European Food Safety Authority (EFSA) have demonstrated that bioactive compounds derived from fermented garlic exert beneficial effects on bone metabolism, growth promotion, and the enhancement of physical fitness and exercise performance (Ried et al., 2025; Lee et al., 2016; El Basuini et al., 2024; Qui et al., 2024). Furthermore, extensive research on protein hydrolysates and bioactive peptides produced through enzymatic hydrolysis of key proteins and sulfur-containing compounds in green garlic has revealed diverse biological activities. These include antioxidant, anti-inflammatory, anticancer, immunomodulatory, antibacterial, antifungal, skin-whitening, and anti-wrinkle effects, alongside high stability, underscoring their potential as functional food and health-promoting ingredients (Arzanlou et al., 2010; Kim et al., 2013; Ezeorba et al., 2024; Marrelli et al., 2025). Collectively, these findings highlight fermentation technology using LAB and QPS-designated strains, applied to regionally distinctive crops such as green garlic, as a promising strategy for developing high-value functional ingredients.
In this study, green garlic with diverse bioactive properties was used to prepare a green garlic fermentation medium. LAB and QPS-designated Bacillus strains were isolated and identified from local specialties of the Sacheon region and subsequently inoculated into the green garlic fermentation medium to produce fermented green garlic. The antioxidant activity of the fermented products was evaluated, and the strain yielding the highest antioxidant activity was selected for further study. The resulting fermented green garlic extract was then assessed for antidiabetic bioactivities. Through this work, we aimed not only to demonstrate the industrial applicability of LAB and QPS-designated Bacillus strains isolated from Sacheon specialties but also to enhance the recognized value of these regional microbial resources.
Materials and Methods
LAB and QPS-designated Bacillus strains were isolated (Table 1) from various local specialty products of the Sacheon region. Sources included Acacia syrup and soy sauce (In-Seok Cheon’s household), pickled perilla leaves (Sang-Gwon Jang’s household), donga makgeolli fermented concentrate (Si-young Seong’s household, Sacheon-eup), ganppang meju doenjang (NH Yonghyeon Agricultural Cooperative, Yonghyeon-myeon), wild kiwi extract soy sauce (Jeong Wol Saem’s farm, Jeongdong-myeon), and gochujang (Kongji Eun’s farm, Sanam-myeon).
| No. | Isolates | Closest match | Similarity (%) | Source | Ref. |
|---|---|---|---|---|---|
| 1 | jo 43-2 | Bacillus amyloliquefaciens | 99.9 | Acacia syrup | Yang et al. (2025) |
| 2 | JS-13-5 | Bacillus amyloliquefaciens | 99.8 | Ganppang meju doenjang | Yang et al. (2025) |
| 3 | JS-65-1 | Bacillus subtilis | 99.7 | Wild kiwi extract soy sauce1) | |
| 4 | JS-66-3 | Bacillus subtilis | 99.9 | Soy sauce3 | Yang et al. (2025) |
| 5 | JY-30-2 | Bacillus subtilis | 99.9 | Pickled perilla leaves | Yang et al. (2025) |
| 6 | JY-48-5 | Bacillus amyloliquefaciens | 99.7 | Gochujang22) | |
| 7 | JY-65-8 | Bacillus subtilis | 99.9 | Wild kiwi extract soy sauce1) | |
| 8 | GH-11-11 | Latilactobacillus curvatus | 100.0 | Sacheon-eup donga makgeolli fermented concentrate | Park et al. (2025) |
| 9 | GH-11-12 | Latilactobacillus curvatus | 100.0 | Sacheon-eup donga makgeolli fermented concentrate | Park et al. (2025) |
| 10 | GH-11-13 | Latilactobacillus curvatus | 100.0 | Sacheon-eup donga makgeolli fermented concentrate | Park et al. (2025) |
| 11 | GH-118-24 | Latilactobacillus curvatus | 100.0 | Sacheon-eup donga makgeolli products | Park et al. (2025) |
Each 1 g sample was suspended in sterile 0.85% saline solution and homogenized using a vortex mixer. One milliliter of the suspension was serially diluted (10–1 to 10–4), and aliquots were spread onto MRS agar and NB agar (BD, USA), prepared as selective media for LAB and QPS-designated Bacillus strains, respectively. Plates were incubated at 37°c, after which colonies were examined for morphological characteristics, including size, shape, and color. Single colonies were then re-streaked on the same medium to obtain pure isolates.
For molecular identification of LAB and QPS-designated Bacillus strains isolated from Sacheon local specialty products, the isolates were cultured on MRS/NB agar plates. 16S rRNA gene sequencing was performed by Macrogen Co., Ltd. (Seoul, Korea). The resulting sequences were analyzed using the 16S-based ID service of EzBioCloud platform (https://www.ezbiocloud.net/), developed and operated by CJ Bioscience, for taxonomic identification.
The production of extracellular hydrolytic enzymes (amylase, lipase, and protease) by the isolated LAB and QPS-designated Bacillus strains was evaluated using substrate-supplemented solid media. Amylase activity was tested on agar plates containing 0.2% soluble starch (BD, USA), lipase activity on plates containing 1% Tween 80 (BD, USA), and protease activity on plates containing 20% skim milk (BD, USA). Each substrate was incorporated individually into MRS/NB agar, which were then inoculated with the isolates and incubated at 37°c for 7 days. Enzyme activity was assessed by measuring the diameter of the clear hydrolysis zones surrounding the colonies. For each isolate, the hydrolytic zone was measured from the edge of the colony to the outer boundary of the clear zone in triplicate or more. The mean values were categorized as follows: +++, >7 mm; ++, 4–6 mm; +, 1–3 mm (Table 2).
Green garlic was wild-harvested in Miryong Village, Sacheon City, and obtained from Il-Geon Lee’s farm. To prepare green garlic powder suitable for fermentation, 500 g of fresh material was sterilized at 121°c for 15 min, dried at 45°c for 48 h, cooled to room temperature, ground into powder, and stored in a sealed container at room temperature until use.
For preparation of the green garlic fermentation medium, 2% (w/v) green garlic powder, 3% (w/v) NaCl, and 0.1% (w/v) yeast extract were mixed with distilled water and sterilized at 121°c for 15 min. Selected LAB and QPS-designated Bacillus strains were pre-cultured in MRS/NB broth, and 1% (v/v) of the pre-culture was inoculated into the green garlic medium. Cultures were incubated at 37°c with shaking at 180 rpm for 4 days, after which the culture supernatant was collected as the fermented green garlic product.
For the preparation of fermented green garlic extract, selected strains were pre-cultured as described above, and 1% (v/v) of the pre-culture was inoculated into 1 L of green garlic medium. Cultures were incubated under previously determined optimal conditions (37°c, 180 rpm, 24–96 h), during which highest antioxidant activity was observed. To extract bioactive compounds, 20 g of Amberlite resins (XAD7HP, XAD4, and XAD16N; Merck KGaA, Darmstadt, Germany) were mixed at a 1:1:1 ratio, washed three times with triple-distilled water, and added to the fully fermented green garlic culture broth. The mixture was shaken at 180 rpm for 2 h to allow adsorption of active compounds onto the resin. The resin was then filtered through cotton cloth and immersed in 150 mL of acetone, followed by shaking at 180 rpm at room temperature for >2 h to desorb the compounds. After filtration through Whatman No. 2 filter paper (Whatman International Ltd., Maidstone, UK), the acetone filtrate was concentrated using a rotary evaporator (Sunileyea, Seongnam, Korea) and finally dried into powder form using a nitrogen gas evaporator (Miulab, Hangzhou, China).
The antioxidant effects of green garlic fermented products produced by each isolated LAB and QPS-designated Bacillus strain were evaluated using ABTS radical scavenging activity. The ABTS radical solution was prepared by mixing 7 mM ABTS with 2.45 mM potassium persulfate (1:1, v/v) and allowing the mixture to stand in the dark for 16 h to generate ABTS radicals (OD=0.70±0.02). For the assay, 20 μL of fermented green garlic product was mixed with 180 μL of ABTS radical solution in a 96-well plate, followed by incubation at room temperature for 2 min. Absorbance was measured at 734 nm, and radical scavenging activity was calculated accordingly.
The antidiabetic effect of the green garlic fermented extract was evaluated by measuring α-glucosidase inhibitory activity using a chromogenic assay (Zhang et al., 2020). In a 96-well plate, phosphate buffer (containing 2 g/L bovine serum albumin and 0.2 g/L NaN3) was mixed with either the green garlic fermented extract or the positive control (acarbose) and incubated for 5 min. The concentrations of both acarbose and the JY-48-5 fermented green garlic sample (extract) used in the experiment were 500 μg/mL. Subsequently, a p-nitrophenyl-α-D-glucopyranoside substrate solution was added, and the mixture was incubated at 37°c for 5 min. The reaction was monitored by measuring absorbance at 405 nm using a microplate reader (Epoch, BioTek, USA). The α-glucosidase inhibitory activity of the samples was then determined from the absorbance values.
The data are represented as the mean±SD of triplicate experiments. The statistical analysis was performed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). The values were evaluated by one-way analysis of variance (ANOVA), followed by a post hoc Duncan’s multiple range test, and values of p<0.05 or p<0.01 were considered statistically significant.
Results and Discussion
LAB have long been used as beneficial microorganisms in fermented foods and continue to attract considerable research attention. They remain widely utilized in the development of functional products aimed at enhancing physiological functions in humans (Wang et al., 2021). In recent years, research has expanded beyond probiotics to include postbiotics, particularly those derived from Bacillus strains recognized under the EFSA Qualified Presumption of Safety (QPS) framework (Hou et al., 2025). In line with these advances, the exploration and identification of novel LAB and QPS-designated Bacillus strains remain active areas of investigation.
In this study, LAB and QPS-designated Bacillus strains were isolated from local special products of the Sacheon region using MRS/NB agar media. A total of 450 strains were obtained from 81 samples of agricultural and marine products. Among these, 191 strains from 39 samples related to agricultural products and their fermented derivatives were identified and characterized (Supplementary Tables 1 and 2). Screening for extracellular enzyme activities identified seven QPS-designated Bacillus strains with strong protease and amylase activities, some of which also displayed lipase activity (Yang et al., 2025). In addition, four LAB strains previously shown to be effective in oyster fermentation (Park et al., 2025) were selected as candidate starter strains for green garlic fermentation.
In total, 11 strains were selected: seven QPS-designated Bacillus strains (from Acacia syrup, soy sauce, pickled perilla leaves, ganppang meju doenjang, wild kiwi extract soy sauce, and gochujang) and four LAB (from donga makgeolli). Based on 16S rRNA gene sequencing, phylogenetic analysis using EzBioCloud identified the isolates as four Latilactobacillus curvatus, three Bacillus amyloliquefaciens, and four Bacillus subtilis strains. These findings not only demonstrate the feasibility of isolating LAB and QPS-designated Bacillus strains from Sacheon specialty products but also highlight the potential of regional resources as valuable reservoirs for discovering diverse strains with safety assurance and multifunctional fermentation capabilities for food applications.
To assess the potential of LAB and QPS-designated Bacillus strains as fermentation starters for high-value food applications, extracellular hydrolytic enzyme activities including protease, amylase, and lipase were evaluated. As summarized in Table 2, all seven QPS-designated Bacillus strains exhibited strong (+++) protease and amylase activities, while three strains also demonstrated detectable (+) lipase activity. Among the LAB strains isolated from donga makgeolli, three showed strong (+++) protease activity and one displayed weaker (+) activity.
Notably, Latilactobacillus curvatus, identified among the isolates, is a registered probiotic strain known to function as a biological protective agent by inhibiting pathogen growth during fermented meat production (Chen et al., 2020). The isolated Bacillus amyloliquefaciens and Bacillus subtilis strains are also included in the most recent EFSA QPS list (EFSA BIOHAZ Panel., 2025), confirming their safety for both food and feed applications. These species are already widely used as functional microorganisms in animal feed and agriculture and possess considerable industrial value, including enzyme production, antimicrobial peptide synthesis, biological control, and use as feed additives. Collectively, the QPS-designated Bacillus strains isolated in this study combine safety assurance with excellent extracellular enzyme activities, highlighting their potential as strategic, high-value microbial resources capable of providing both functional versatility and industrial applicability in food fermentation.
Recently, an increasing number of studies have reported that fermentation of green garlic with LAB and QPS-designated Bacillus strains enhances its physiological activities. In this study, the isolated strains were evaluated as fermentation starters with potential to augment the inherent bioactivities of green garlic, which is naturally rich in allicin, niacin, β-carotene, and vitamins A, B1-B3, C, and K. These compounds are associated with antioxidant, anti-inflammatory, immune-enhancing, antibacterial, antifungal, skin-whitening, anti-wrinkle, and anti-aging effects (Arzanlou et al., 2010; Kim et al., 2013; El Basuini et al., 2024; Ezeorba et al., 2024; Qui et al., 2024; Marrelli et al., 2025; Ried et al., 2025). Green garlic was processed into powder and incorporated into the fermentation medium, after which selected strains with strong protease, amylase, and lipase activities were inoculated and cultured. Culture supernatants were collected daily as fermented products.
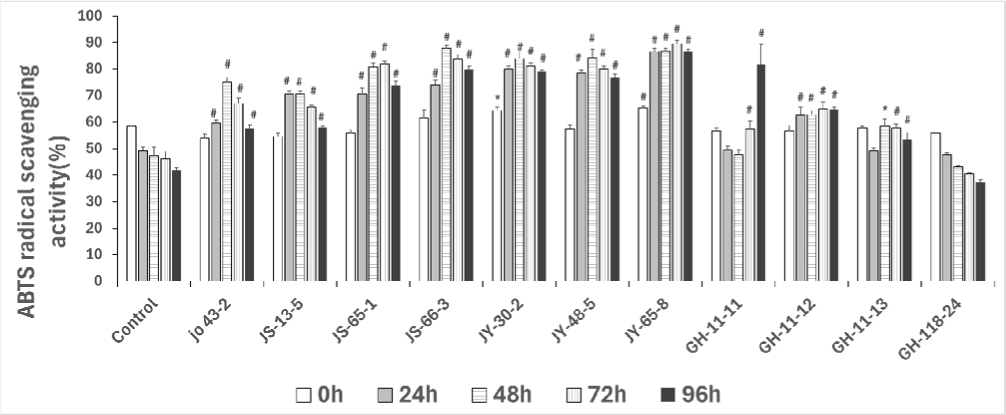
Antioxidant activity, measured by ABTS radical scavenging, revealed that green garlic fermented with B. amyloliquefaciens JY-48-5, B. subtilis JS-65-1, and Latilactobacillus curvatus GH-11-11 exhibited significantly higher activity compared to the non-fermented control. The strongest effects were observed in JY-48-5 (145.8% at 2 days), JS-65-1 (144.9% at 2 days), and GH-11-11 (144.0% at 4 days) (Fig. 1).
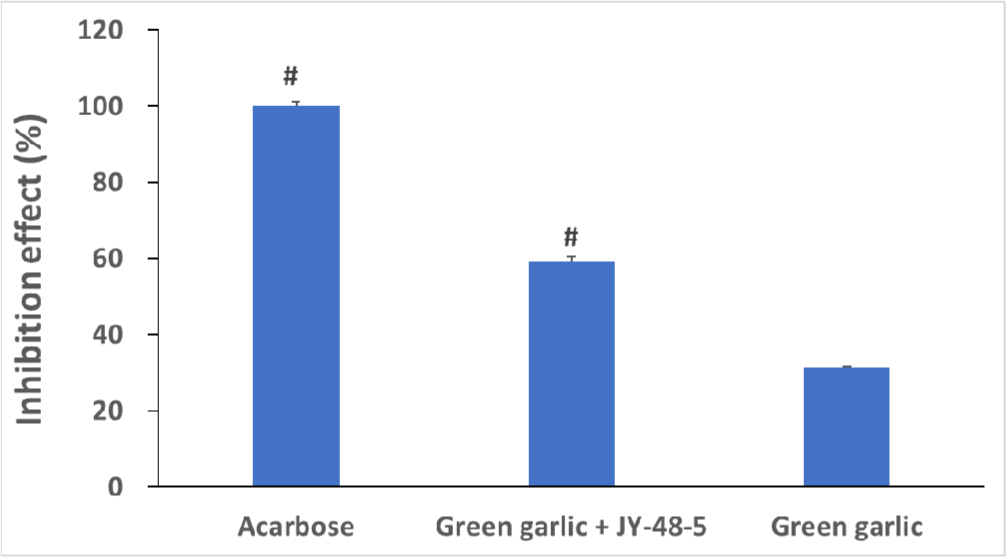
Given these results, the strain with the highest antioxidant capacity, B. amyloliquefaciens JY-48-5 (2-day fermentation), was selected for further study. The resulting fermented extract was assessed for antidiabetic potential by measuring α-glucosidase inhibitory activity, a key mechanism for regulating blood glucose. The extract showed a 189.4% increase in inhibitory activity compared to the non-fermented extract. Notably, it exhibited approximately 59% of the inhibitory effect of acarbose, a clinically prescribed α-glucosidase inhibitor for type 2 diabetes (Fig. 2). These findings suggest that green garlic fermented extract can delay glucose release by inhibiting intestinal α-glucosidase, thereby contributing to reduced plasma glucose levels.
This study provides the first report in Korea on the antioxidant and antidiabetic effects of green garlic fermented with LAB and QPS-designated Bacillus strains isolated from Sacheon local specialties. The results highlight the industrial potential of these fermented extracts as functional ingredients for food and cosmetic applications. Moreover, the strains identified in this study have been deposited in the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Biological Resource Center and the Sacheon Microbial Fermentation Foundation, ensuring their availability as valuable microbial resources.







