Introduction
Rice (Oryza sativa L.) is a staple food and one of the most commonly consumed cereals in Asia (Chung & Shin, 2007; Li et al., 2022). As the physiological activity of rice has been reported, interest in specialty rice has been steadily increasing (Loan et al., 2024). Among the types of specialty rice, colored rice is highly beneficial to health with improved quality as it contains various minerals, vitamins, and polyunsaturated fatty acids (PUFA) (Rathna Priya et al., 2019; Mbanjo et al., 2020). In addition, it has a special flavor and pigments, such as tannins and anthocyanins. Anthocyanins are a type of flavonoid and water-soluble pigments that appear in red, purple and blue colors. They have the effect of reducing the risks related to oxidation, inflammation, and cardiovascular diseases (Seo et al., 2008; Tena et al., 2020).
Black rice bran, a byproduct of grain refining, is rich in various components, including phytic acid, vitamin E, polyphenols, γ-oryzanol, and hemicelluloses, as well as anthocyanins (Das et al., 2023). While black rice bran is an abundant source of nutritional components, it has the disadvantage of being difficult to digest (Kong & Lee, 2010).
When rice bran is fermented with fungi, enzymes secreted by the microorganisms break down the complex structure of the rice bran, leading to an increase in phenolic acid content. During fermentation, microbial metabolism produces important compounds such as organic acids, phenolic acid, and polyunsaturated fatty acids (PUFA) (Oliveira et al., 2011; Shin et al., 2019). Additionally, hydrolytic enzymes from microorganisms, such as those derived from probiotics, degrade high molecular-weight polymers such as carbohydrate and protein weaken molecular bonds, and modify the structure of substrates, thereby increasing digestibility. Given these advantages, the fermentation process is used for biotechnological application (Park & Oh, 2005; Tu et al., 2021).
Most studies on rice bran fermentation focus on fungi (Schmidt et al., 2014), with relatively few investigating Bacillus spp. According to Li et al. (2023), Bacillus strains commonly found in traditional fermented foods secrete enzymes such as amylase and protease, enhancing the nutritional content. These strains also produce extracellular polysaccharides and peptides with antimicrobial properties that inhibit harmful microorganisms. Despite these benefits, the use of Bacillus remains limited by antimicrobial substances inhibition from black rice. Research on rice bran fermentation has also been limited for colored rice, and it has only recently begun to attract attention. Therefore, this study aims to ferment black rice bran using Bacillus spp. derived from traditional Korean fermented foods and analyze the nutritional contents of the fermented black rice bran extract.
Materials and Methods
Black rice bran was obtained by milling black rice harvested in Jincheon, Korea. It was dried in an oven (OF12GW, Jeio-Tech Co., Seoul, Korea) at 60°C for 10 h. The dried black rice bran was milled using a blender (Blender 7012S, Waring, Torrington, CT, USA) and stored at 4°C.
Eighteen strains of Bacillus spp. were previously isolated from traditional Korean fermented foods, Kimchi and Gochujang, in our laboratory. Each strain was preserved at –80°C in glycerol stock (20% v/v) and sub-cultured in tryptic soy broth (TSB; Difco Laboratories, Detroit, MI, USA) prior to use in experiments.
To screen Bacillus strains for black rice bran fermentation, amylolytic and proteolytic activities were evaluated using the protocol described by Medeiros et al. (2018). For amylolytic activity, the strains were sub-cultured in TSB and then streaked on TSB agar containing 1% soluble starch. The plates were incubated at 37°C for 2 days. A 1% Lugol’s iodine solution was poured onto the TSB agar plates, and the presence of a clear zone surrounding the colony indicated amylolytic activity.
To evaluate the proteolytic activity, the strains were sub-cultured in TSB and then streaked on 1% skim milk agar. The plates were incubated at 37°C for 48 h. A clear zone surrounding the colony indicated proteolytic activity. These activities were quantified by measuring the diameter of the clear zone surrounding the colony.
Among 18 Bacillus spp., five strains were selected for the fermentation of black rice bran. Black rice bran medium was prepared by adding black rice bran to distilled water at a 4% (w/v) ratio and autoclaving the mixture at 121°C for 15 min. The selected Bacillus spp. were sub-cultured in TSB and then transferred to the black rice bran medium at an inoculation rate of 2% (v/v). The culture was incubated at 37°C for 24 h with shaking at 150 rpm. During incubation, samples were taken at 0, 4, 8, 12, and 24 h. The samples were serially diluted with 0.1% sterile peptone water, and the appropriate dilutions were plated onto TSB agar plates. The viable cell was counted after 24 h of incubation at 37°C.
To analyze the nutritional components of fermented black rice bran, the black rice bran supernatant was filtered using Whatman No. 2 filter paper. Non-fermented black rice bran, collected at 0 h fermentation, was filtered through a filter paper. The filtered extracts were freeze-dried and stored at –20°C until further analysis.
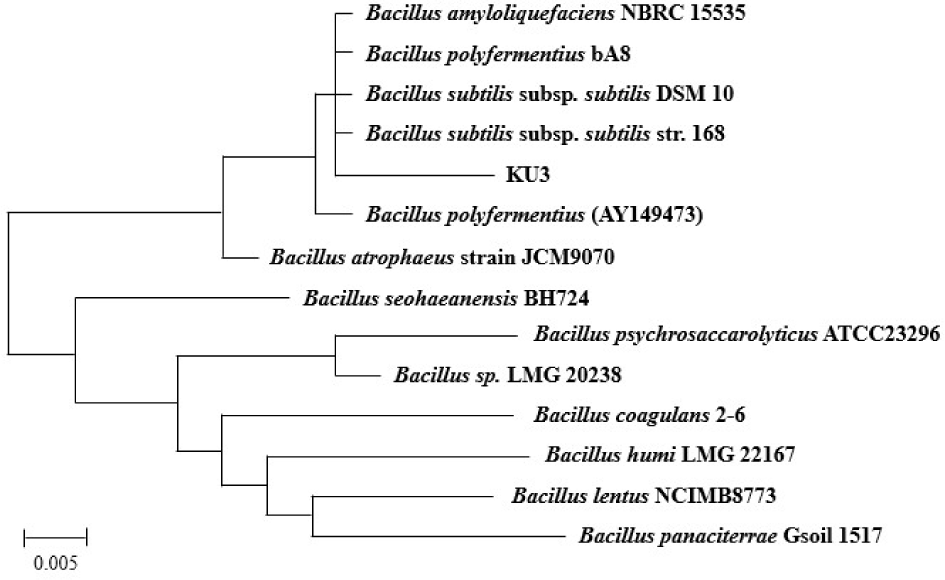
The selected Bacillus spp. was identified using an API 50 CHB kit (BioMerieus, Lyon, France). The result was analyzed using biochemical profiles of the apiwebTM database. The 16S rRNA sequencing analysis was conducted by Macrogen (Seoul, Korea). The obtained sequence was compared to the NCBI (National Center for Biotechnology Information) data base using the BLAST software. A phylogenetic tree was then constructed using MEGA software and Clustal X for alignment.
The nutritional components (moisture, total ash, lipid, and nitrogen content) of black rice bran and fermented black rice bran extracts were analyzed using the methods outlined by the Association of Official Analytical Chemists (AOAC, 1998). Total carbohydrate content was calculated by subtracting other components.
Statistical analysis was conducted using the SPSS 18 software (Chicago, IL, USA). Mean values were statistically analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests and Students-t test. The p-values less than 0.05 were considered statistically significant.
Results and Discussion
Eighteen Bacillus spp. were previously isolated from Korean traditional fermented foods (Kim et al., 2012). These isolates have often used as fermentation starters, as reported in previous studies (Lee et al., 2010; Lim, 2010). Among them, Bacillus spp. produce various enzymes, which contribute to hydrolysis of substrates during fermentation (Alrahmany et al., 2013; Li et al., 2023). Accordingly, the amylolytic and proteolytic activities of Bacillus spp. indicate the ability to break down the components of black rice bran during fermentation. Therefore, the amylolytic and proteolytic activities of Bacillus spp. were measured to screen Bacillus strains, and the results are shown in Table 1. Only four of the 18 Bacillus spp. showed amylolytic activity on starch agar. The KU3 and KU28 strains exhibited the highest amylolytic activity, having clear zones of 5 mm. With regard to proteolytic activity, all 18 of the Bacillus spp. showed clear zones of between 4 and 26 mm on the skim milk agar. Six of the 18 Bacillus spp. had clear zones of over 16 mm. The KU24 strain exhibited the largest clearing zone. KU2 and KU28 strains also had high clear zones over 20 mm. Ten of the Bacillus spp. had clear zones between 8 and 16 mm. Only two of the strains had clear zones of less than 8 mm. Among the 18 Bacillus spp., four strains (KU3, KU28, KU611, and KU612) had both amylolytic and proteolytic activities. With four strains, KU24, which had the highest proteolytic activities, was selected as primary strains for further fermentation of black rice bran.
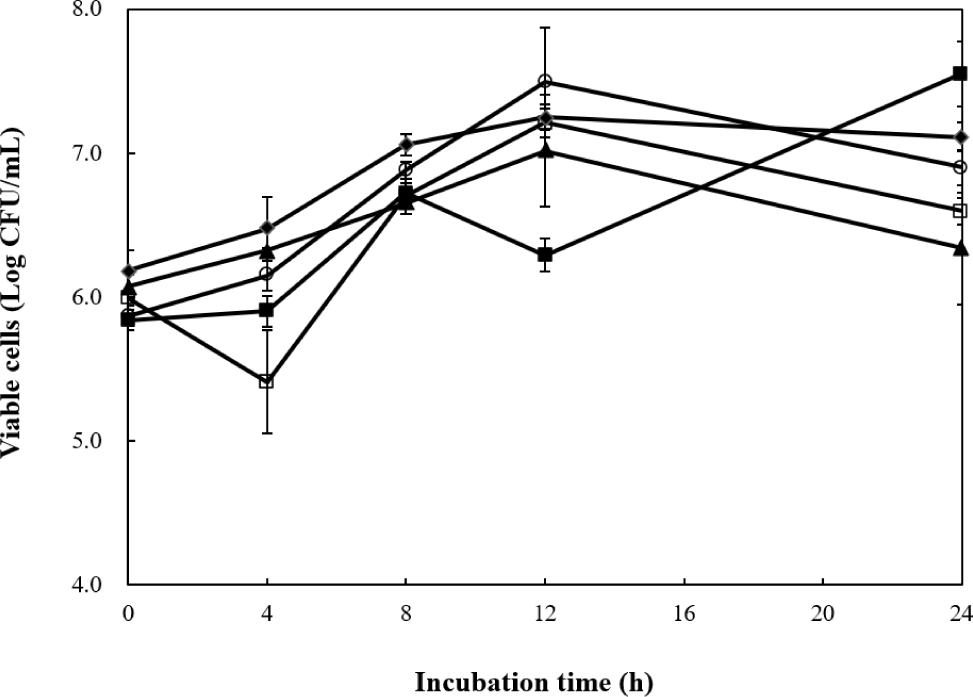
Five selected Bacillus strains (KU3, KU24, KU28, KU611, and KU612) were grown in black rice bran. The numbers of viable cells in black rice bran medium are presented in Fig. 1. The initial viable cell counts of the five selected strains ranged from 5.83 to 6.18 Log CFU/mL. The viable cell number of KU3 strain had the highest ability to grow in black rice bran medium, increasing from 5.83 to 7.55 Log CFU/mL after 24 h. Viable cell numbers of KU24, KU28, KU611, and KU612 strains gradually increased for 12 h and decreased after 24 h of fermentation. The viable cell numbers of KU24 and KU28 strains increased from 5.86 and 5.99 Log CFU/mL to 7.49 and 7.21 Log CFU/mL, respectively, after 12 h of fermentation. The cell numbers decreased to 6.89 and 6.59 Log CFU/mL, respectively, after 24 h. Kim et al. (2006) reported that Bacillus strains and lactic acid bacteria were well grown during fermentation of brown rice. The viable cell numbers of Bacillus strains increased more than 8 Log CFU/mL after 24 h or 48 h (Kim et al., 2006). The better growth observed in brown rice is due to its inclusion of the germ and endosperm, making it nutritionally richer than black rice bran and therefore, more suitable for the growth of Bacillus strains. In a study on fermentation of black rice bran with lactic acid bacteria, black rice bran was hydrolyzed using commercial α-amylase and then fermented with lactic acid bacteria. Although viable cell count was not analyzed, the pH decreased from approximately 6.0 before fermentation to around 3.4 after 24 h of incubation, suggesting that lactic acid bacteria grew in hydrolyzed black rice bran (Lin et al., 2024).
The extraction yields of non-fermented black rice bran and fermented black rice bran for 24 h by selected strains (KU3, KU24, KU28, KU611, and KU612) are shown in Table 2. The extraction yields of non-fermented black rice bran ranged from 17.46 to 18.18. After 24 h of fermentation, the extraction yields increased by approximately 5%, from 22.02 to 23.96%. The extraction yield of fermented black rice bran by the KU611 strain was the highest. However, there was no significant difference in the extraction yields of fermented black rice bran by other four strains.
Among the selected strains, KU3 strain exhibited both amylolytic and proteolytic activities, and showed a trend of increasing viable cell count after 24 h fermentation. It was therefore considered to have a higher survival rate in black rice bran medium compared to other strains. Thus, KU3 strain was considered as the best strain for fermentation of black rice bran. This KU3 strain was identified as Bacillus subtilis/amyloliquefaciens (98.5% similarity) by biochemical fermentation patterns in Table 3. In addition, the strain showed 99.9% similarity with Bacillus subtilis based on partial sequence comparison using BLAST. The result of the 16S rRNA sequencing of Bacillus subtilis KU3 is shown in Fig. 2.
The results of the proximate analysis for each extract are shown in Table 4. The carbohydrate was shown to be the primary constituent of both black rice bran extracts with the contents of 77.26% and 77.13%. Only the ash content increased after fermentation. The lipid contents of both black rice bran extracts were lower than that reported in other studies on rice bran. This is likely due to water being used as the solvent, which results in lower lipid components (Kupski et al., 2012; Gosangi & Sharma, 2023). Choi et al. (2010) and Faccin et al. (2009) reported that lipid and carbohydrate contents decreased while protein, ash, and moisture contents increased after fermentation of rice bran for 2 weeks. Ribeiro et al. (2024) reported that there were changes in the composition over the fermentation period. As fermentation by fungi progressed, the carbohydrate content decreased, and consequently, the content of other components increased. During the fermentation of soybeans with Bacillus spp., the degradation of proteins and carbohydrates was observed, accompanied by a significant increase in peptides and oligosaccharides (Amoa-Awua et al., 2006). These results suggest that the degradation of carbohydrate and protein progressed during fermentation with Bacillus subtilis (KU3 strain) which exhibited both amylolytic and proteolytic activities.
| Components | Non-fermented black rice bran extract | Fermented black rice bran extract |
|---|---|---|
| Carbohydrate | 77.26±0.21 | 77.13±0.12 |
| Protein | 10.89±0.24 | 10.06±0.22 |
| Lipid | 2.02±0.10 | 1.70±0.15 |
| Ash | 9.80±0.03 | 11.14±0.33 |
In conclusion, Bacillus spp. isolated from Korean traditional fermented foods screened for the fermentation of black rice bran. The KU3 strain identified as Bacillus subtilis was the potential strain as a starter culture for the fermentation of black rice bran with increasing the availability of nutrients. Additionally, this study provided the possible utilization of black rice bran through fermentation to develop functional food ingredients. It will need to investigate the physiological activity of fermented black rice bran as further study.