Introduction
Probiotics have become a popular topic in health research because of their ability to modulate gut microbiota, enhance immune responses, and alleviate gastrointestinal disorders (Bodke & Jogdand, 2022; Chandrasekaran et al., 2024). Benefits of probiotics include alleviating the symptoms of irritable bowel syndrome, improving lactose digestion, and contributing to mental health by modulating the gut-brain axis (Didari et al., 2015; Ansari et al., 2023). However, the clinical efficacy of probiotics is highly dependent on their viability during production, storage, and gastrointestinal transit (Bernatek et al., 2022). Maintaining high viability remains a major challenge because of the susceptibility of probiotic cells to environmental stressors, such as freeze-drying, oxidative stress, and the acidic or bile-rich conditions of the gastrointestinal tract (Wendel, 2022).
Conventional encapsulation technologies, including alginate- and chitosan-based coatings, provide some degree of mechanical protection, but often fail to mitigate oxidative and osmotic stress during freeze-drying (Yeung et al., 2016; Pupa et al., 2021). Moreover, these methods lack scalability and cost-effectiveness, which are critical for commercial production (Agriopoulou et al., 2023). Recent advancements have explored the incorporation of antioxidant compounds, including polyphenols and synthetic molecules, into nanoencapsulation systems. However, concerns regarding cost, regulatory approval, and probiotic strain compatibility have limited their widespread adoption (Pateiro et al., 2021). There is an urgent need for a multifunctional coating system that enhances mechanical stability and mitigates oxidative and osmotic stress, ensuring high probiotic viability throughout the product lifecycle.
To overcome the limitations of conventional encapsulation techniques and enhance probiotic stability, this study aims to evaluate the effectiveness of VitaShield Coating® (VSC) as a novel stabilization strategy (BIFIDO, 2024). VSC employs a lipid-based coating system enriched with vitamins A, C, and E, providing a dual-action mechanism. This approach improves oxidation resistance by minimizing oxygen penetration and reduces osmotic stress through better hydration control. In contrast to conventional microencapsulation, VSC features a streamlined process suitable for large-scale production. Functional materials were selected based on their biocompatibility, antioxidant properties, and ability to maintain probiotic viability, positioning VSC as a scalable and effective alternative.
The industrial application of probiotics requires the careful selection of strains that demonstrate both functional efficacy and stability under varying environmental conditions. Bifidobacterium bifidum BGN4 was selected for this study due to its well-established safety profile, proven bio-functional effects, and long-standing commercial use. Supported by extensive scientific evidence, this study investigates the potential of VSC technology to enhance the viability and stability of B. bifidum BGN4 during production, storage, and gastrointestinal transit.
Materials and Methods
B. bifidum BGN4 obtained from BIFIDO Co. Ltd. (Korea) was cultured in De Man, Rogosa, and Sharpe (MRS) broth (BD Difco™, USA) supplemented with 0.05% (w/v) cysteine hydrochloride (Sigma-Aldrich, USA) to enhance anaerobic growth. Cultivation was performed in an anaerobic chamber (Coy Laboratory Products, USA) at 37°C for 18 h. Following incubation, cells were harvested by centrifugation using a model 5810R centrifuge (Eppendorf, Germany) at 5,000×g for 10 min at 4°C. The resulting pellet was washed twice with sterile phosphate-buffered saline (PBS; pH 7.4, Gibco™, USA) and resuspended in PBS to achieve a final cell density of approximately 109 colony forming units (CFU)/mL, determined using a Neubauer counting chamber.
VSC Formulation
VSC solutions were prepared by emulsifying pharmaceutical-grade retinyl acetate (Vitamin A; Sigma-Aldrich, USA) and α-tocopherol (Vitamin E; Sigma-Aldrich, USA), as both constituents are poorly water-soluble. Emulsification was facilitated using polysorbate 80 (Tween 80; Sigma-Aldrich, USA) as a hydrophilic surfactant. The vitamins were mixed with the surfactant at a final concentration of 0.1–0.5% (w/v) and homogenized using a probe sonicator for 10–15 min to achieve a stable dispersion. Ascorbic acid (Vitamin C; Sigma-Aldrich, USA), which is highly water-soluble, was dissolved in sterile distilled water. The vitamin solutions were then combined at a 3:2:1 weight ratio and optimized through preliminary trials to ensure efficient coating and probiotic viability. The final mixture was thoroughly blended to form a homogeneous coating.
B. bifidum BGN4 probiotic cells were harvested at the late exponential growth phase by centrifugation at 4,000×g for 10 min at 4°C, followed by two washes with sterile PBS to remove residual medium. The collected cells were gently mixed with VSC solution at a 1:1 (v/v) ratio and incubated by gentle stirring at 4°C for 30 min to ensure uniform coating.
The coated probiotic suspension was freeze-dried to obtain a stable powder. The suspension was evenly spread (∼3-mm thick) on sterile trays and frozen at –80°C for 12 h to ensure complete solidification. Frozen samples were subsequently transferred to a FreeZone® 6 freeze dryer (Labconco, USA) set at –40°C and 0.1 mbar. The shelf temperature was gradually increased to –30°C over a period of 6 h to facilitate ice sublimation while preserving cell viability. Residual moisture was removed during secondary drying by increasing the shelf temperature to 20°C and maintaining a vacuum of 0.05 mbar for 8 h, reducing the final moisture content to <5%. The resulting freeze-dried powder was collected under sterile conditions, stored in moisture-proof containers with silica gel desiccants, and maintained at 4°C to ensure the stability and viability of the coated B. bifidum BGN4. This process effectively preserved probiotic viability and maintained the functional integrity of the VSC coating.
Experimental Protocols
To evaluate freeze-drying recovery, coated and uncoated B. bifidum BGN4 cells were pre-frozen at –40°C in a deep freezer (Thermo Fisher Scientific, USA) for 12 h. The samples were freeze-dried using a FreeZone Freeze Dryer (Labconco, USA) under vacuum (0.01 mbar) for 24 h. Viable cell counts before and after drying were determined using the pour plate method with MRS agar (BD Difco™, USA), incubated anaerobically at 37°C for 48 h. The recovery rate (%) was calculated as:
To assess bacterial resilience during gastrointestinal transit, coated and uncoated B. bifidum BGN4 were sequentially exposed to simulated gastric fluid (SGF; pH 2.0) and bile salt solution. SGF was prepared by dissolving pepsin (Sigma-Aldrich, USA) in 0.1 N HCl, while the bile salt solution consisted of 0.3% (w/v) oxgall (Difco™, USA) in sterile distilled water. Samples were incubated separately in each solution at 37°C for 2 h under gentle agitation (100 rpm) using a New Brunswick Innova 40 Incubator Shaker (Eppendorf, Germany). After incubation, viable cell counts were determined by plating 100 µL aliquots on MRS agar, followed by anaerobic incubation at 37°C for 48 h.
Membrane lipids were extracted using the method of Bligh and Dyer. Briefly, 5 mL of the cell suspension was mixed with a chloroform-methanol solution (2:1, v/v; Sigma-Aldrich, USA) and agitated for 10 min. After phase separation, the organic phase containing lipids was collected, evaporated under nitrogen gas (Parker Balston, USA), and stored at –20°C until analysis. The extracted lipids were transesterified into fatty acid methyl esters (FAMEs) by incubating the mixture at 60°C for 30 min using a lipid-to-methanol ratio of 1:4 (v/v) with 2% sulfuric acid in high-performance liquid chromatography-grade methanol (Sigma-Aldrich, USA). After incubation, the reaction mixture was centrifuged at 10,000×g for 10 min, and the supernatant containing FAMEs was collected for gas chromatography-mass spectrometry (GC-MS) analysis. The GC-MS analysis was carried out using a model 7890A GC instrument coupled with a model 5975C MS detector (Agilent Technologies, USA) and equipped with a DB-23 capillary column (60 m×0.25 mm×0.25 µm; Agilent, USA). The injection temperature was maintained at 250°C, while the detector temperature was set at 280°C. Helium served as the carrier gas with a steady flow rate of 1.0 mL/min. The oven temperature program began at 50°C, held for 1 min, then increased to 175°C at a rate of 25°C/min, followed by a further increase to 230°C at a rate of 4°C/ min, where it was held for 5 min. Depending on the sample concentration, injections were performed in either split or splitless mode. The fatty acid composition was identified by comparing mass spectra with the national institute of standards and technology (NIST) mass spectral library and retention time data of standard FAME mixtures. Based on this analysis, the fatty acid profiles, including saturated, monounsaturated, and polyunsaturated fatty acids (PUFAs), were quantified to evaluate the impact of VSC on membrane fluidity and structural resilience.
To evaluate the effect of VSC coating on the storage stability of probiotic strains, four Bifidobacterium strains (B. lactis AD011, B. longum BORI, B. bifidum BGN4, and B. longum RAPO) were selected and stored under two different temperature and humidity conditions. Both coated and uncoated samples were sealed in sterile aluminum stick packs and stored under the following conditions:
The samples were placed in temperature- and humidity-controlled incubators (Memmert GmbH, Germany) and maintained for a total duration of 16 weeks. To monitor bacterial viability over time, samples were retrieved at predetermined intervals (weeks 0, 2, 4, 6, 8, 12, and 16) and analyzed to assess the impact of VSC coating on the long-term storage stability of probiotic strains. The percentage of viable cell retention was calculated by normalizing the CFU counts to the initial CFU count at week 0, allowing for a comparative analysis of survival rates between coated and uncoated samples stored under different temperature conditions.
All experiments were performed in triplicate. The data are expressed as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test, with a significance threshold of p<0.05. Statistical analyses were performed using the GraphPad Prism 9 software (GraphPad Software, USA).
Results
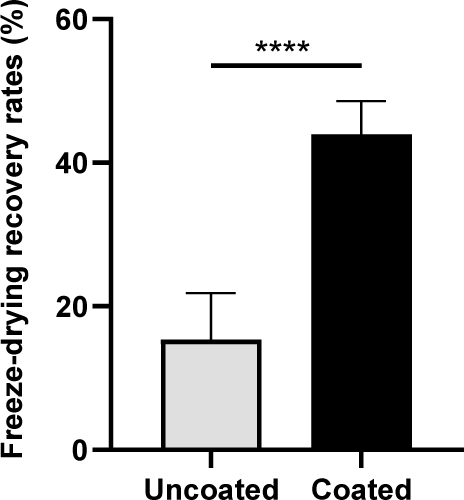
The freeze-drying recovery rates of coated and uncoated cells were compared to evaluate the protective efficacy of the coating material during lyophilization. Coated cells exhibited a significantly higher freeze-drying recovery rate than uncoated cells (15.31±6.53% vs. 43.91±4.69%, respectively; p<0.0001) (Fig. 1). This substantial improvement highlights the protective role of the coating in preserving cellular integrity during freeze-drying.
Scanning electron microscopy (SEM) revealed that coated cells maintained structural integrity with minimal morphological disruption. In contrast, uncoated cells displayed clear evidence of membrane collapse and surface shrinkage (Fig. 2). These findings corroborate the quantitative recovery results and suggest that the coating mitigated the mechanical and osmotic stresses during freeze-drying.

The gastrointestinal stability of coated and uncoated cells was assessed in SGF and bile solution. In SGF, coated cells retained 26.20±2.41% viability, significantly outperforming the 3.20±2.30% viability rate of uncoated cells (p<0.0001). Similarly, in bile solution, the viability of coated cells significantly exceeded the rate of uncoated cells (57.94±2.73% vs 35.04±2.98%; p<0.0001) (Fig. 3).

GC-MS analysis revealed a significant increase in PUFAs in coated cells. The unsaturated to saturated fatty acid ratio in coated cells was 1.5:1, compared to 1:2 in uncoated cells. Specifically, PUFAs increased from 12.50±0.63% in uncoated cells to 28.70±0.75% in coated cells (p<0.0001) (Fig. 4). This enhanced PUFA content likely contributed to the observed improvements in freeze-drying recovery, gastrointestinal stability, and storage performance.

VSC coating significantly enhanced the storage stability of Bifidobacterium strains under different temperature conditions. To evaluate the protective effect of VSC coating, the viability of four probiotic strains (AD011, BORI, BGN4, and RAPO) was analyzed under 25°C and 30°C conditions over a 16-week storage period. The results demonstrated that VSC coating effectively improved the storage stability of all strains, with the coated group exhibiting significantly higher viability than the uncoated group at week 16. This finding suggests that VSC treatment mitigates viability loss over time, thereby providing a protective effect during prolonged storage.
Throughout the storage period, the viability trends of coated and uncoated samples remained similar in the early stages. However, as the storage period progressed, notable differences emerged between the two groups, particularly under 30°C conditions. For BGN4, at week 6, the coated sample stored at 30°C exhibited a higher survival rate than the uncoated sample stored at 25°C, indicating that VSC coating contributes to maintaining probiotic viability even under elevated temperatures (Fig. 5C). Similarly, RAPO at week 16 showed greater viability in the coated group at 30°C compared to the uncoated group at 25°C, further demonstrating the protective effect of VSC treatment over an extended period (Fig. 5D).

These results suggest that VSC coating not only enhances probiotic stability but also reduces the adverse effects of elevated storage temperatures. The observation that coated samples stored at 30°C maintained viability levels comparable to or exceeding those of uncoated samples stored at 25°C highlights the potential of VSC treatment in protecting probiotic strains from environmental stress.
Discussion
This study highlights the considerable advantages of the VSC technology in improving the stability and viability of the B. bifidum BGN4 probiotic during essential processes, such as freeze-drying, gastrointestinal transit, and storage. These findings emphasize the multifunctional protective mechanisms of VSC, which concurrently alleviate mechanical, osmotic, and oxidative stresses, and ultimately enhance survival and efficacy of the probiotic bacteria.
The superior freeze-drying recovery of VSC-coated cells demonstrates the efficacy of the coating in preserving cellular integrity. SEM analysis confirmed that VSC protects against structural damage, ensuring morphological stability in coated cells. In contrast, uncoated cells exhibit membrane collapse and surface shrinkage. Structural preservation is crucial because membrane integrity directly influences viability and metabolic functionality following lyophilization. Moreover, the lipid composition of the cellular membrane is a critical determinant of resilience to freeze-drying stress (Cui et al., 2022). GC-MS analysis revealed a significantly increased ratio of unsaturated to saturated fatty acids in the coated cells, with a notable increase in PUFAs. Such lipid remodeling is likely to enhance membrane fluidity, mitigate rigidity-induced stress, and prevent membrane rupture during dehydration and rehydration cycles (Lee et al., 2020).
We observed that the protective effects of VSC extend beyond freeze-drying and significantly enhances gastrointestinal stability. In SGF and bile solutions, VSC-coated cells exhibited significantly higher survival rates than uncoated cells. This enhanced resistance to harsh physiological conditions is attributed to the hydrophobic barrier formed by retinyl acetate (Vitamin A), which shields the cellular membrane from acid-induced denaturation and osmotic stress (Sun, 2012). Additionally, the elevated PUFA content in coated cells likely improves membrane adaptability under acidic and bile-rich conditions by increasing bilayer flexibility, thereby mitigating lipid peroxidation and membrane destabilization. These findings are consistent with those of previous studies indicating that an increased PUFA-to-saturated fatty acid ratio enhances probiotic resilience in the gastrointestinal tract by preserving membrane integrity under stress (Marrone & Coccurello, 2019; Hutchinson et al., 2020).
The prolonged storage stability of VSC-coated B. bifidum BGN4 cells further underscores their industrial significance. Over extended storage periods, coated cells exhibited greater viability than uncoated cells, particularly at elevated temperatures. This improved stability suggests that VSC can address the logistical challenges associated with probiotic distribution, particularly in regions with variable climatic conditions. This stabilization effect is likely facilitated by α-tocopherol (Vitamin E), which prevents lipid peroxidation and sustains membrane integrity during prolonged storage (Niki, 2021; Sattler et al., 2004). Additionally, the modified fatty acid profile in VSC-coated cells may contribute to thermal stability by preventing phase transitions that compromise membrane functionality at high temperatures. This observation is consistent with a previous description that increased PUFA levels enhance thermotolerance in probiotic strains by modulating membrane dynamics and reducing oxidative damage (Yeung et al., 2016).
The observed increase in PUFAs in VSC-coated cells may be attributed to the protective role of the antioxidant components within the coating matrix. Vitamins A, C, and E are known to scavenge free radicals and inhibit lipid peroxidation, which can help preserve unsaturated fatty acids during storage. In particular, vitamin E has a well-established role in stabilizing membrane lipids, and vitamin C can regenerate oxidized vitamin E, enhancing the overall antioxidant defense. Furthermore, the lipid-based structure of the VSC may contribute to maintaining membrane fluidity and stability, thereby indirectly supporting the retention of PUFAs. Although further studies are required to elucidate the precise molecular mechanisms, these findings suggest that VSC plays a protective role in preserving membrane lipid composition under environmental stress conditions.
Compared with conventional encapsulation methods, VSC exhibits distinct advantages. Although alginate-based systems are effective in providing a moisture barrier, they offer limited protection against oxidative stress and exhibit structural degradation during freeze-drying. Similarly, chitosan coatings and polyphenol-enhanced matrices possess protective properties and lack the multifunctional capabilities of VSC. The ability of VSC to simultaneously counteract multiple stressors makes this approach a superior alternative for probiotic stabilization.
Despite these promising findings, some limitations of this study must be acknowledged. The efficacy of VSC was evaluated using a single probiotic strain. The performance of VSC across strains with diverse structural and metabolic properties remains to be explored. Although GC-MS analysis provides critical insights into changes in lipid composition, further research is needed to elucidate the precise molecular mechanisms by which VSC interacts with cellular membranes to enhance stability. Moreover, this study was performed under in vitro conditions, necessitating validation through in vivo assessments to determine the influence of the coating on probiotic colonization and health benefits. Future investigations should focus on broadening the scope of probiotic strains, optimizing freeze-drying conditions, and integrating additional functional additives such as prebiotics to enhance the commercial viability of VSC. Understanding the molecular pathways affected by the coating components will further support the development of next-generation probiotic stabilization technologies.
Conclusion
The collective findings of this study document the substantial advantages offered by VSC as a robust, scalable, and multifunctional strategy for enhancing probiotic stabilization and viability. This approach has considerable potential for the industrial implementation and formulation of high-performance probiotic products by effectively mitigating the critical challenges associated with freeze-drying, gastrointestinal transit, and long-term storage. Further investigations are warranted to expand the applicability of VSC and comprehensively elucidate the underlying mechanisms.







