Introduction
Edible films have gained significant attention in the food industry, particularly for their application in extending the shelf life of high-moisture fresh produce, such as fruits and vegetables (Pavlath & Orts, 2009; Singh et al., 2022; Chavan et al., 2023). These films function by preserving freshness through multiple mechanisms, including physical protection, moisture retention, respiration control, prevention of oxidation, and inhibition of microbial growth. In response to the growing global concerns about health and environmental pollution caused by plastic waste, the development of biodegradable edible films made from natural biopolymers with properties such as biocompatibility, biodegradability, and non-toxicity has gained increasing importance (Umaraw & Verma, 2017). These films not only reduce the use of synthetic preservatives and additives but also contribute to the development of environmentally friendly biodegradable plastics.
Cellulose nanofiber (CNF), derived from cellulose, the most abundant natural biopolymer on Earth, is characterized by its high aspect ratio, with diameters ranging from 5 to 30 nm and lengths in the micrometer scale, and by containing both crystalline and amorphous regions (Poulose et al., 2022). CNF is typically produced by removing hemicellulose and lignin through enzymatic and chemical pretreatments, followed by mechanical processes such as high-pressure homogenization, microfluidization, micro grinding, steam explosion, cryo-crushing, and high-intensity ultrasonication, which reduce the fibers to the nanoscale (Nadeem et al., 2022; Poulose et al., 2022). Due to its high tensile strength and transparency, low oxygen permeability, excellent thermal stability, and biodegradability, CNF has been considered a promising material for use in biodegradable food packaging (Habibi et al., 2010; Pakharenko et al., 2021; Basumatary et al., 2022). However, limitations such as poor water solubility and relatively low elongation properties have necessitated research into blending CNF with other natural biopolymers or plasticizers to improve its film-forming capabilities (Azeredo et al., 2009; Yu et al., 2017; Alves et al., 2019; Pirozzi et al., 2021).
Shellac (Sh) is a water-insoluble natural resin secreted by the insect Laccifer lacca, primarily found in Southeast Asia and China. It is classified as GRAS (generally recognized as safe) by the U.S. Food and Drug Administration and is widely used in the food industry for applications such as film/coatings for preserving fresh produce, glazing agents, oil-gel formers, and foaming agents (Yuan et al., 2021; Thombare et al., 2022; Kumar et al., 2023). Sh is a low-molar-mass resin primarily composed of oxyacid polyester, where aleuritic acids and cyclic terpene acids are linked through esterification (Yuan et al., 2021). Six types of cyclic terpene acids, including jalaric, shellolic, laksholic, laccijalaric, laccishellolic, and laccilaksholic acids, have been identified (Yuan et al., 2021). Sh has a highly complex chemical structure, with unique and intricate properties that vary depending on factors such as the raw material, type of acid, and degree of esterification (Ahuja & Rastogi, 2023). Sh has excellent film-forming properties and unique pH-responsive characteristics, therefore, it has been studied for the formation of films or coatings in combination with plasticizers (e.g., polyethylene glycol), organic acids (e.g., oleic acid), polysaccharides (e.g., pectin), and proteins (Luangtana-Anan et al., 2017; Yuan et al., 2021; Ahuja & Rastogi, 2023).
Recently, studies on CNF-Sh composite films have been also reported. Basic properties of the films formed by blending non-edible CNF extracted from straw by chemical treatments with Sh at varying concentrations (0–40% w/w) were reported (Mohamed et al., 2022). Another study examined the basic properties of films formed through the polyesterification of aleuritic acid, a component of Sh, after blending with CNF at ratios ranging from 0% to 20% (w/w) (Tedeschi et al., 2020). In this study, composite films were prepared by blending Sh with CNF extracted from oats through enzymatic pretreatment and high-pressure homogenization, and the physical and mechanical properties of these films were analyzed to explore their potential application as edible films or coatings.
Materials and Methods
CNF was obtained from the Korea Textile Machinery Convergence Research Institute (Gyeongsan, Korea), and its preparation is briefly described as follows. Dietary fiber from German oats was hydrolyzed using three commercial enzymes, including Viscozyme® Wheat FG, LAMINEX® Super 3G, and Viscoflow® MG, in which cellulase and xylanase were contained. Subsequently, the enzyme was inactivated by heating at 100°C for 30 min, followed by high-pressure homogenization (NH 4000, Ilshin Autoclave Co., Ltd., Korea) to prepare a CNF dispersion containing 1.5% total solid. Sh was purchased as edible dewaxed bleached shellac powder from Shellac Korea (Busan, Korea). NaHCO3, glycerol, and Mg(NO3)2 were obtained from Junsei Chemical Co. Ltd. (Tokyo, Japan), Daejung Chemicals and Metals (Siheung, Korea), and Samchun Pure Chemical Co., Ltd. (Seoul, Korea), respectively.
The CNF dispersion (1.5% total solid) was sterilized at 121°C for 15 min and then stored at 4°C until use. The Sh solution was prepared by adding Sh powder to a 0.3 M NaHCO3 aqueous solution at a concentration of 10% (w/w), homogenization (T25 digital Ultra-Turrax, IKA, Königswinter, Germany) at 10,000 rpm for 3 min, followed by stirring at 500 rpm for 10 min at 60°C. The prepared Sh solution was stored at 4°C until use.
The CNF dispersion and Sh solution were mixed at CNF:Sh (w/w) ratios of 100:0, 50:50, 20:80, and 0:100, and designated as CNF, Sh50, Sh80, and Sh100, respectively. The total solid content of all mixtures was fixed at 2% (w/w), with glycerol (0.6%, w/w) added as a plasticizer. The mixtures were stirred at 60°C for 10 min, then homogenized at 10,000 rpm for 3 min, and used immediately after preparation.
CNF-Sh composite films were formed by pouring each prepared mixture (25 mL) into a polytetrafluoroethylene-coated Petri dish (i.d., 12 cm), followed by drying at 35°C for 24 h. The formed films were then removed from the Petri dish and conditioned in a desiccator containing a saturated Mg(NO3)2 solution at 53% relative humidity (RH) at 25°C for at least 48 h before further experiments.
The thickness of the films was measured using a digital micrometer (BD293-025, Bluetec, Shanghai, China). For each type of film, 10 samples were prepared, and the thickness was measured at 10 different locations per sample, resulting in an average value from a total of 100 measurements.
The opacity of the films was measured using a UV-Spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The films were cut into 1×4 cm specimens, and transmittance (T) was measured at a wavelength of 600 nm. The opacity was then calculated using equation (1). For each film type, three samples were prepared, and the opacity was measured at 10 different locations per sample, resulting in an average value from 30 measurements (Zhao et al., 2022).
where x = film thickness.
The moisture content of the films was determined by the gravimetric method according to AOAC (2000) with a slight modification. The films were cut into 2×2 cm specimens, dried at 105°C for 48 h, and the mass difference before and after drying was obtained to calculate moisture content. For each film type, an average value from 10 measurements was recorded.
The water solubility of the films was determined according to the method of Dordevic et al. (2023) with some modifications. The films were cut into 2×2 cm specimens, dried at 105°C for 24 h, and their mass (M1) was recorded. The dried films were then immersed in 50 mL of distilled water at room temperature for 24 h. The films were then collected, dried again at 105°C for 24 h, and their mass (M2) was measured. The water solubility of the films was calculated as follows. For each film type, an average value from 10 measurements was obtained.
The swelling of the films was determined according to the methods of Torstensen et al. (2022) and Wang et al. (2023) with some modifications. The films were cut into 2×2 cm specimens and placed in a desiccator containing a saturated Mg(NO3)2 solution (RH 53%) at 25°C for 48 h, after which their mass (M53) was recorded. The films were then transferred to a desiccator containing a saturated NaCl solution (RH 75%) and stored at 25°C for another 48 h before measuring their mass (M75). The swelling was calculated as follows. For each film type, an average value from 10 measurements was obtained.
The contact angle (θ) of the films was measured using a contact angle goniometer (Phoenix-MT(M), Surface Electro Optics, Suwon, Korea), according to the methods of Gao et al. (2018), Zhao & Jiang (2018), and Mostafavi (2019) with some modifications. The films were cut into 2×2 cm specimens, and the measurements were conducted by placing a 20 μL droplet of triple-distilled water onto the film surface at 20°C and 40% RH. For each film type, five samples were prepared, and the contact angle was measured at five different locations per sample, resulting in an average value from 25 measurements.
The mechanical properties of the films were analyzed at 25°C using a texture analyzer (TA1, Ametek Lloyd Instruments Ltd., Largo, FL, USA) according to the methods of Dai et al. (2017) and Lindström (2021) with some modifications. The films were prepared in 1×4 cm specimens, and the stress-strain curve for each film was obtained, with an initial grip length of 10 mm, a preload of 0.05 N, and a testing speed of 3 mm/min. The tensile strength and elongation at break were calculated using the following equations.
where τ = stress at break (N), A = the initial cross-sectional area of the film (m2), Δl = elongation distance at break (mm), and l0 = the initial grip separation distance (10 mm).
The yield stress (MPa) of the films was obtained as the stress value at the point where strain begins to increase. Young’s modulus (MPa) was calculated as the slope of the initial linear region (strain<0.01) of the stress-strain curve. The work of break (MJ/m3) was determined by integrating the area under the stress-strain curve up to the point of breakage. All values were averaged from measurements of at least 40 film samples.
Data were expressed as the mean±standard deviation. Data were analyzed by one-way analysis of variance using IBM SPSS Statistics (version 26.0, IBM Co., Armonk, NY, USA). Duncan’s multiple range test was used to determine significant differences between data at a confidence level of p≤0.05.
Results and Discussion
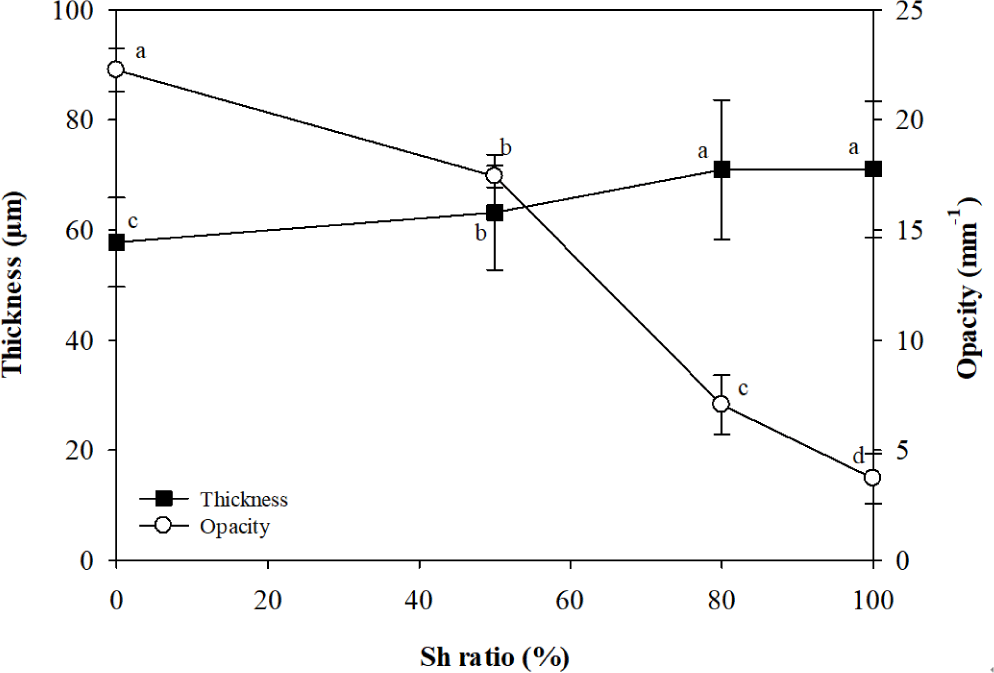
The thickness of the CNF film was 57.8 μm, and it increased to 63.2 μm and 71.0 μm as the content of Sh increased to 50% and 80%, respectively. The film composed of Sh only (Sh100) had a thickness of 71.1 μm, which was similar to that of Sh80 (Fig. 1). The increase in thickness with increasing Sh content is likely due to the increased moisture content and swelling as Sh is added (Fig. 2). A similar trend was also reported in a study on composite films of konjac glucomannan and Sh, where the authors attributed the thickness increase with Sh content to the increased chain stiffness resulting from interactions between the two biopolymers (Du et al., 2019).
The opacity of the films showed a significant decreasing trend with increasing Sh content, with the opacity of Sh50, Sh80, and Sh100 films being 78%, 32%, and 17% of that of the CNF film, respectively (Fig. 1). This decrease is likely due to the reduction in the content of crystalline CNF, which contributes to light scattering, alongside an increase in the content of Sh. Sh does not form crystals because of its complex structure, in which aleuritic acids and cyclic terpene acids are linked through esterification (Yuan et al., 2021).
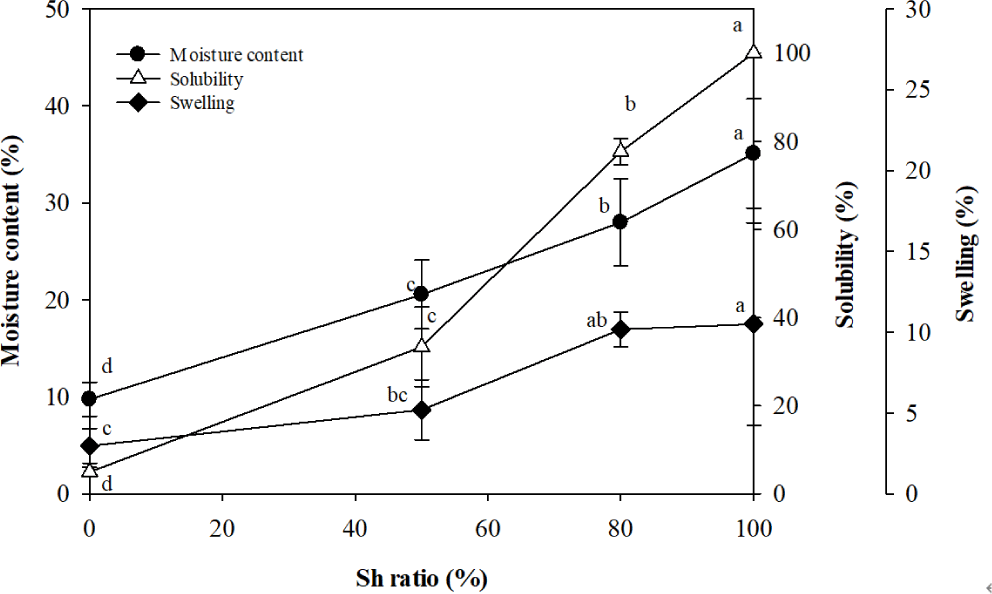
For moisture content, the CNF film exhibited a value of 9.7%, but as the Sh content increased, the moisture content significantly rose, with the Sh100 film showing a 3.6-fold higher value of 35.1% (Fig. 2). Regarding solubility, the CNF film displayed a low value of 4.9%, but the solubility significantly increased with higher Sh content, with the Sh50 and Sh 80 films reaching 33.3% and 77.7%, respectively, and the Sh100 film being completely dissolved in water (Fig. 2). As for swelling, the CNF film showed a low value of 3%, but the swelling increased as the Sh content increased, with the Sh80 and Sh100 films showing values of 10.2% and 10.5%, respectively (Fig. 2).
These results indicate that the hydrophilicity of the films increases with increasing Sh content. In this study, Sh powder was dissolved in a NaHCO3 aqueous solution to prepare a Sh solution for composite films. During the dissolution process, it is assumed that the ester bonds in Sh were cleaved, increasing the number of carboxyl groups (Limmatvapirat et al., 2004; Chen et al., 2024). Thus, the addition of Sh likely enhanced the hydrophilicity of the films by increasing the amount of carboxyl groups, which have a high affinity for water.
The CNF and Sh50 films exhibited contact angles of 38.2° and 39.3°, respectively, with no significant difference between the two values. These relatively low contact angles indicate high hydrophilicity of the film surfaces. In contrast, the Sh80 and Sh100 films initially displayed larger contact angles of 67.4° and 80.6°, respectively, immediately after the droplet was placed. However, within 10 s, the surface of the films began to swell, leading to an inability to maintain the initial contact angle. The hydrophobic nature of aleuritic acid, a component of Sh, may be responsible for the increased initial contact angles of Sh80 and Sh100 films (Yuan et al., 2021). However, as previously discussed, increasing the Sh content enhances the concentration of hygroscopic carboxyl groups, facilitating the dissolution of Sh into the droplet, which may lead to the rapid swelling observed on the surfaces of Sh80 and Sh100 films upon contact with the droplet.
Representative examples of the stress-strain curves for the four types of films are shown in Fig. 3a, demonstrating significant differences in curve shape depending on the Sh content. For tensile strength, the CNF film exhibited a value of 17.91 MPa, which decreased as the Sh content increased, with the Sh100 film showing a very low value of 0.3 MPa (Fig. 3b). Regarding elongation at break, the CNF film displayed a low value of 10%, while the elongation increased with increasing Sh content, reaching 253% for the Sh100 film (Fig. 3b). Yield stress, Young’s modulus, and work of break of the films also significantly decreased with increasing Sh content. The reduction in Young’s modulus indicates a decrease in film stiffness, and the reduction in work of break suggests a decrease in film toughness (Table 1).
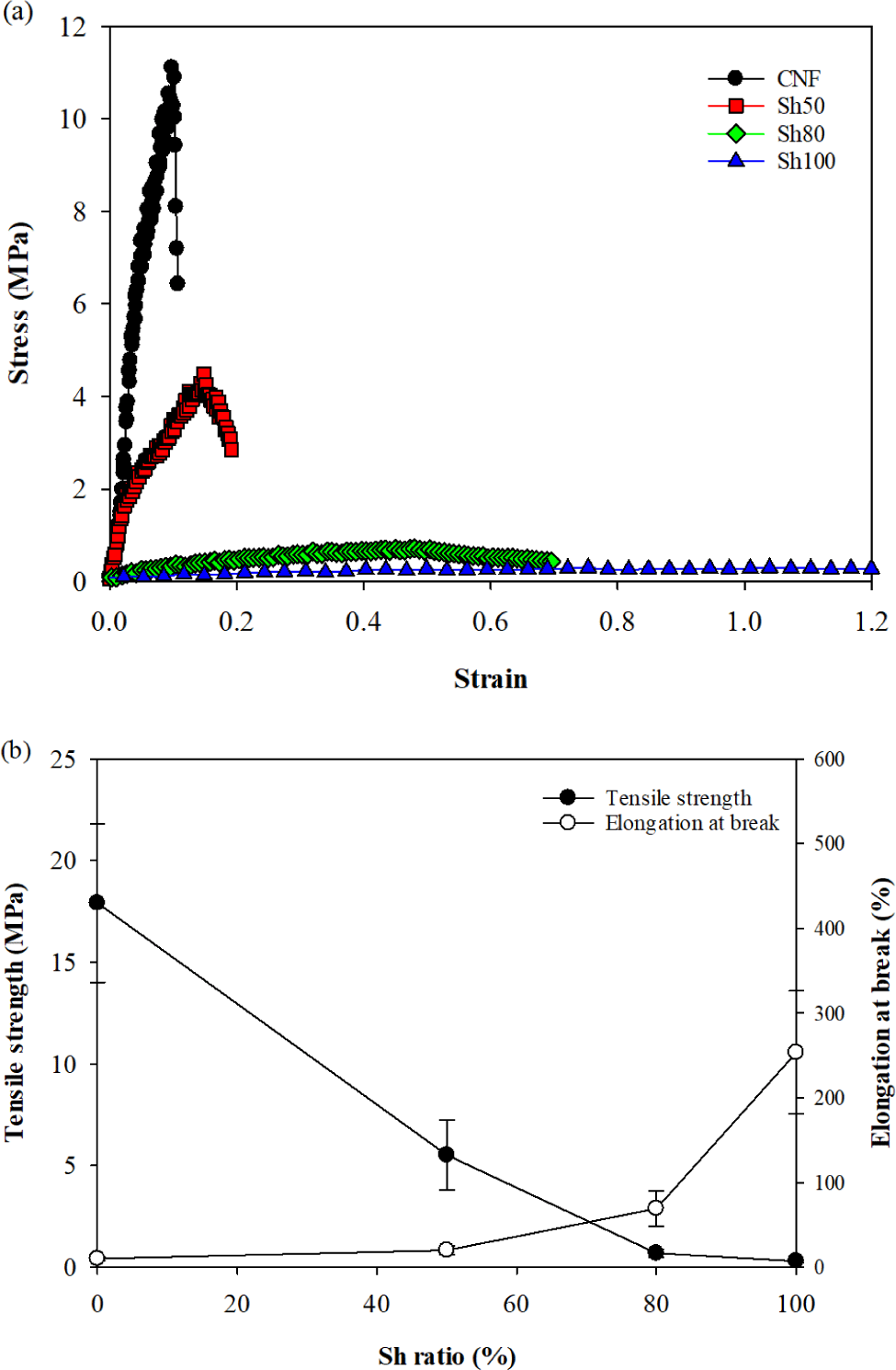
The CNF film is anticipated to form a robust network structure with low moisture content due to the high stiffness of CNF chains arising from their crystalline structure. Sh consists of smaller molecules compared to CNF, and the cleavage of ester bonds in the alkaline solution further reduced its molar mass. As a result, the small Sh molecules can act as a plasticizer, disrupting the formation of a compact CNF network. Additionally, the water molecules introduced by Sh function as a plasticizer. These factors may enhance the flexibility of the film, thereby reducing its stiffness, brittleness, and toughness. However, a study on composite films of Sh and konjac glucomannan reported that both tensile strength and elongation at break increased with the addition of Sh (Du et al., 2019). This was attributed to the esterification between the carboxyl groups of Sh and the hydroxyl groups of konjac glucomannan
Conclusion
This study demonstrated that blending Sh with CNF enables the production of composite edible films with varied thickness, opacity, moisture-related properties, and mechanical characteristics. CNF film was relatively thin, highly opaque, possessed low moisture content, and exhibited a rigid structure that resisted dissolution and swelling in water, with low elongation. The surface of CNF film was hydrophilic; however, the rigid bonds and crystalline structure of CNF likely contribute to its low responsiveness to moisture. As the Sh content increased, there was a corresponding increase in the thickness, moisture content, solubility, swelling, and elongation at break of the films. In contrast, opacity, tensile strength, yield stress, Young’s modulus, and work of break decreased significantly. This behavior can be attributed to the enhanced plasticity resulting from the incorporation of hydrophilic Sh with relatively low molar mass, coupled with a reduction in crystalline CNF, which typically contributes to light scattering and rigid network structure. Edible films (or coatings) should be transparent to minimize visual rejection, adhere well to uneven food surfaces without peeling during drying, form a robust coating layer with minimal sensory impact, and provide effective moisture and oxygen barrier properties while dissolving quickly during washing. This study suggests that the combination of CNF, which forms a compact network structure, and Sh, which contributes plasticity, flexibility, and transparency, enables the production of edible films with adjustable properties tailored for specific purposes.