Introduction
Alcohol is one of the most widely consumed central nervous system depressants in human history. A hangover is a complex of symptoms occurring after excessive alcohol intake, including headache, nausea, vomiting, loss of appetite, diarrhea, chills, cold sweats, cognitive impairment, and mood swings. It may also involve reduced recognition, exercise capacity, hematological abnormalities, and hormonal changes. Hangovers are a common phenomenon among individuals who consume alcohol and may cause physical discomfort, reduce work capacity, and result in social and economic loss (Karadayian & Cutrera, 2013; Lv et al., 2022; Kim et al., 2023).
The absorption and metabolism of alcohol play an important role in human physiology and pathology. After oral ingestion, alcohol passes through the oral cavity and esophagus and reaches the stomach, where it stimulates the mucosa. High concentrations of alcohol can induce mucosal damage and inflammation, which may promote gastric acid secretion as a defense response (Bode & Bode, 1997; Franke et al., 2005). Approximately 20% of alcohol is absorbed in the stomach, and the majority is absorbed in the small intestine. This absorption ratio depends on gastric emptying rate, alcohol concentration, and co-ingestion of food. Most of the absorbed alcohol is transported to the liver via the portal vein, where first-pass metabolism occurs. After metabolism, alcohol is distributed to other tissues through systemic circulation (Hyun et al., 2021; Stevens et al., 2022). Meanwhile, Meanwhile, unmetabolized alcohol is distributed through the bloodstream and used as an energy source or excreted via respiration, urine, and sweat. The final metabolic products of alcohol are carbon dioxide and water, and the elimination rate varies depending on liver function, body weight, sex, and the amount of alcohol consumed (Cederbaum, 2012).
The liver is the central organ for alcohol metabolism, processing about 90% of ingested alcohol. Alcohol is first oxidized to acetaldehyde by alcohol dehydrogenase (ADH). In response to chronic or excessive alcohol intake, the activity of the microsomal ethanol oxidizing system, primarily mediated by cytochrome P450 2E1, increases to facilitate the elevated metabolic flux of ethanol. This system generates oxidative stress and promotes the accumulation of toxic metabolites in the liver (Lieber, 2004; Lu & Cederbaum, 2008; Jiang et al., 2020). In the second metabolic step, acetaldehyde is converted into acetate by aldehyde dehydrogenase (ALDH). Acetaldehyde is a toxic intermediate that, without efficient removal, may lead to hepatocellular injury, fatty liver, inflammation, and fibrosis, which contribute to the development of alcohol-related liver diseases such as alcoholic hepatitis and cirrhosis (Edenberg, 2007; Liu et al., 2021; Xu et al., 2025). Furthermore, chronic or excessive alcohol consumption can increase the risk of cardiovascular conditions such as hypertension and arrhythmia (Piano, 2017; Arora et al., 2022). Overall, alcohol affects not only the central nervous system but also multiple organ systems, including the gastrointestinal tract, liver, and cardiovascular system (Pervin & Stephen, 2021).
To alleviate hangover symptoms resulting from acetaldehyde accumulation and associated effects of alcohol consumption, various products have been developed to support alcohol metabolism and relieve related symptoms. Thus, this study evaluated the metabolic effects of the Morning Care series—including PRESSON G, PRESSON H2, PRESSON I, and EX PREMIUM—on ethanol and acetaldehyde metabolism in a mouse model by measuring the activities of ADH and ALDH, as well as analyzing blood concentrations of ethanol and acetaldehyde.
Material and Methods
Morning Care PRESSON G (20060445110881), Morning Care PRESSON H2 (20060445110880), Morning Care PRESSON I (20060445110933), and Morning Care EX PREMIUEM (20080181270198) were provided from Dong-A Pharm(Seoul, Korea). Each product includes a number assigned by the regulatory authority when a food manufacturing or processing business reports the production of an item. The detailed list of ingredients for each product is shown in Table 1.
The male and female C57BL/6J mice (6-week-old, 16–18 g) were purchased from ORIENT BIO Inc. (Seongnam, Korea) and caged at a controlled temperature of 23±2°C, 55±10% relative humidity, 12 h light/dark cycles, and freely fed commercial feed (ORIENT BIO Inc.) and tap water ad libitum for 1 week. After a period of acclimation, mouse was divided into the following 5 groups (n=12 for each group): group 1 (water administration, ethanol feeding), group 2 (Morning Care H from Dong-A Pharm company administration, ethanol feeding), group 3 (Morning Care PRESSON H administration, ethanol feeding), group 4 (pro Morning Care PRESSON G administration, ethanol feeding), and group 5 (Morning Care EX administration, ethanol feeding). All experimental groups were orally administered one of the Morning Care products—PRESSON G, PRESSON H2, PRESSON I, or EX PREMIUM—followed by ethanol administration (10 mL/kg body weight) after 30 minutes. The blood and liver from the mouse were obtained 1, 3, or 5 h after ethanol feeding. The animals were kept in a specific pathogen-free environment. All the animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Hanyang University (protocol 2024-0257) and were performed in accordance with Korean Food and Drug Administration guidelines.
Five hundred microliters of the blood from mouse were taken and centrifuged at 3,500×g for 10 min and stored at –80°C until assayed. The alcohol concentration in serum was measured using the Ethanol Bio HT (Roche, Darmstadt, Germany).
Alcohol dehydrogenase activity assay kit and ALDH activity colorimetric assay kit (Sigma-Aldrich Co., St. Louis, MO, USA) were used to analyze the activity of ADH and ALDH in mouse liver. Two hundred microliters of ADH buffer or ALDH buffer were added to 100 mg of mouse liver, and mouse liver tissues were destroyed by ultrasonicating for 5 s using sonicator (Vibra cell, SONICS, Newtown, CT, USA) followed by centrifugation at 13,000×g for 10 min. The supernatant was used to determine ADH and ALDH activity. One unit of ADH or ALDH is the amount of enzyme that generates 1.0 μmole of NADH per minute at pH 8.0 at 37°C (for ADH) or room temperature (for ALDH).
To determine the expression level of ADH and ALDH genes in mouse liver, 2 mg of liver tissue was destroyed using a sonicator and the RNA extracted using RNeasy Mini Kit (QIAGEN, Hilden, Germany). The cDNA was synthesized by the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) and the FastStart Essential DNA Green Master (Roche) was used as the master mix for real-time PCR. The primers used were designed as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH; housekeeping gene, 5′-ATC CCA TCA CCA TCT TCC AG-3′; forward primer, 5′-CCT GCT TCA CCA CCT TCT TG-3′; reverse primer), ADH (5′-ACC ATC GAG GAC ATA GAA-3′; forward primer, 5′- GTG GAG CCT GGG GTC AC-3′; reverse primer), and ALDH2 (5′-GCT GTC AGC AAG AAA ACA TTC CCC-3′; forward primer, 5′-CTT GTC AGC CCA GCC AGC ATA ATA-3′; reverse primer). The real-time PCR conditions for the amplification of these genes were an annealing temperature of 58°C and the cycle number was 45. The real-time PCR machine used was the QuantStudioTM 3 (ABI; Thermo Fisher Scientific Inc., Waltham, MA, USA) and primers were synthesized by Macrogen (Seoul, Korea).
The data were determined with mean values and standard deviations in triplicate trials. Statistical analyses were performed using SPSS 23 (SPSS Inc., Chicago, IL, USA). Differences between multiple groups were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference post hoc test for multiple comparisons. A p<0.05 was considered statistically significant.
Results
To evaluate the effect of the Morning Care series on blood ethanol and acetaldehyde concentrations, male and female mice were tested at 1, 3, and 5 hours after ethanol feeding. Blood concentrations of ethanol and acetaldehyde were measured at each time point and are presented in Fig. 1. In male mice, no significant difference in blood ethanol concentrations was observed at 1 hour following administration of any Morning Care product. At 3 hours, ethanol concentrations were significantly reduced by all treatments compared to the vehicle, with PRESSON I, PRESSON H2 and EX PREMIUM showing the most pronounced reductions. This reduction was maintained at 5 hours, where ethanol levels remained lowest in PRESSON I, PRESSON H2 and EX PREMIUM. A similar trend was observed in female mice. While no significant differences were found at 1 hour, blood ethanol concentrations were significantly reduced at 3 and 5 hours in the sample-treated groups compared to the vehicle group. At 3 hours, PRESSON I and EX PREMIUM significantly lowered ethanol concentrations compared to the vehicle. At 5 hours, all Morning Care treatments significantly reduced ethanol levels compared to the vehicle, but no significant difference was observed among the treatments (Fig. 1A).
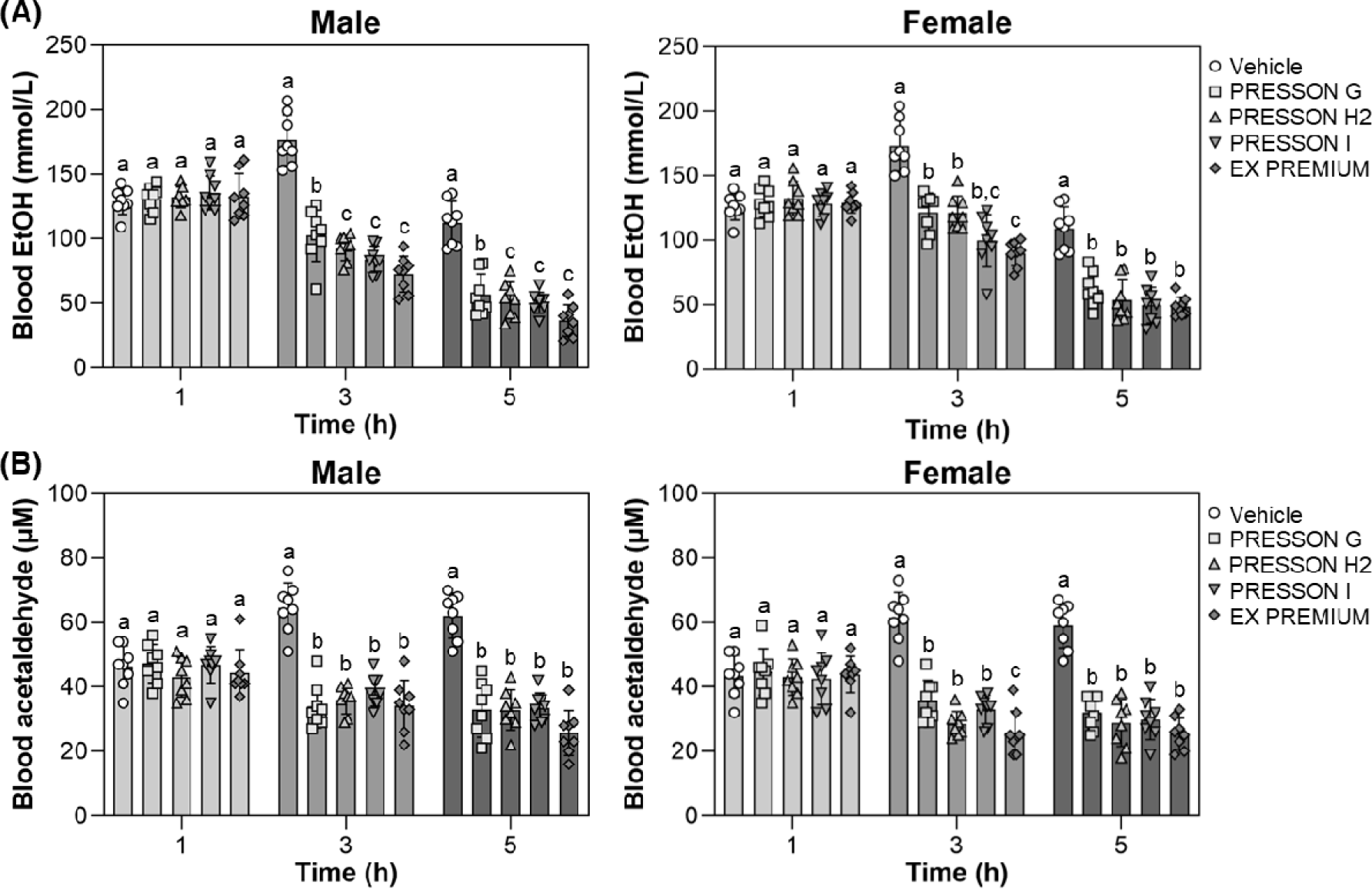
Blood acetaldehyde concentration, a key metabolite of ethanol, was measured to assess the metabolic response to alcohol intake. In male mice, there was no significant difference in blood acetaldehyde concentrations between the sample-treated groups and the vehicle group at 1 hour. However, at 3 hours, all sample-treated groups showed a significant reduction in blood acetaldehyde levels compared to the vehicle group, and this reduction was maintained at 5 hours. A similar trend was observed in female mice. While no significant differences were found at 1 hour, blood acetaldehyde concentrations were significantly reduced at 3 and 5 hours in all treatment groups compared to the control. At 3 hours, EX PREMIUM produced the greatest reduction in acetaldehyde concentration. At 5 hours, all treatments resulted in comparable reductions, each significantly lower than the vehicle (Fig. 1B).
Overall, the Morning Care series effectively reduced blood ethanol and acetaldehyde concentrations over time in both sexes. EX PREMIUM showed the most pronounced reductions at several time points, particularly at 3 and 5 hours, supporting their potential role in enhancing aldehyde clearance and alleviating hangover symptoms.
To examine the effect of sample treatments on acetaldehyde metabolism, ADH activity was measured in the blood and liver of male and female mice at 1, 3, and 5 hours after ethanol feeding, which was administered 30 minutes following sample treatment. In male mice, blood ADH activity at 1 hour was significantly elevated in all treatment conditions compared to the vehicle. At 3 hours, EX PREMIUM showed significantly higher ADH activity than vehicle and other treatments, and this increased activity was maintained at 5 hours (2.88 nmole/min/mg), although the difference among the treatments was not statistically significant (Fig. 2A). In female mice, blood ADH activity was significantly increased at all time points in all Morning Care-treated conditions compared to vehicle. At 1 and 3 hours, EX PREMIUM exhibited significantly higher activity than the other Morning Care series, and at 5 hours, EX PREMIUM maintained the highest level (3.13 nmole/min/mg), which remained significantly elevated compared to vehicle (Fig. 2A).
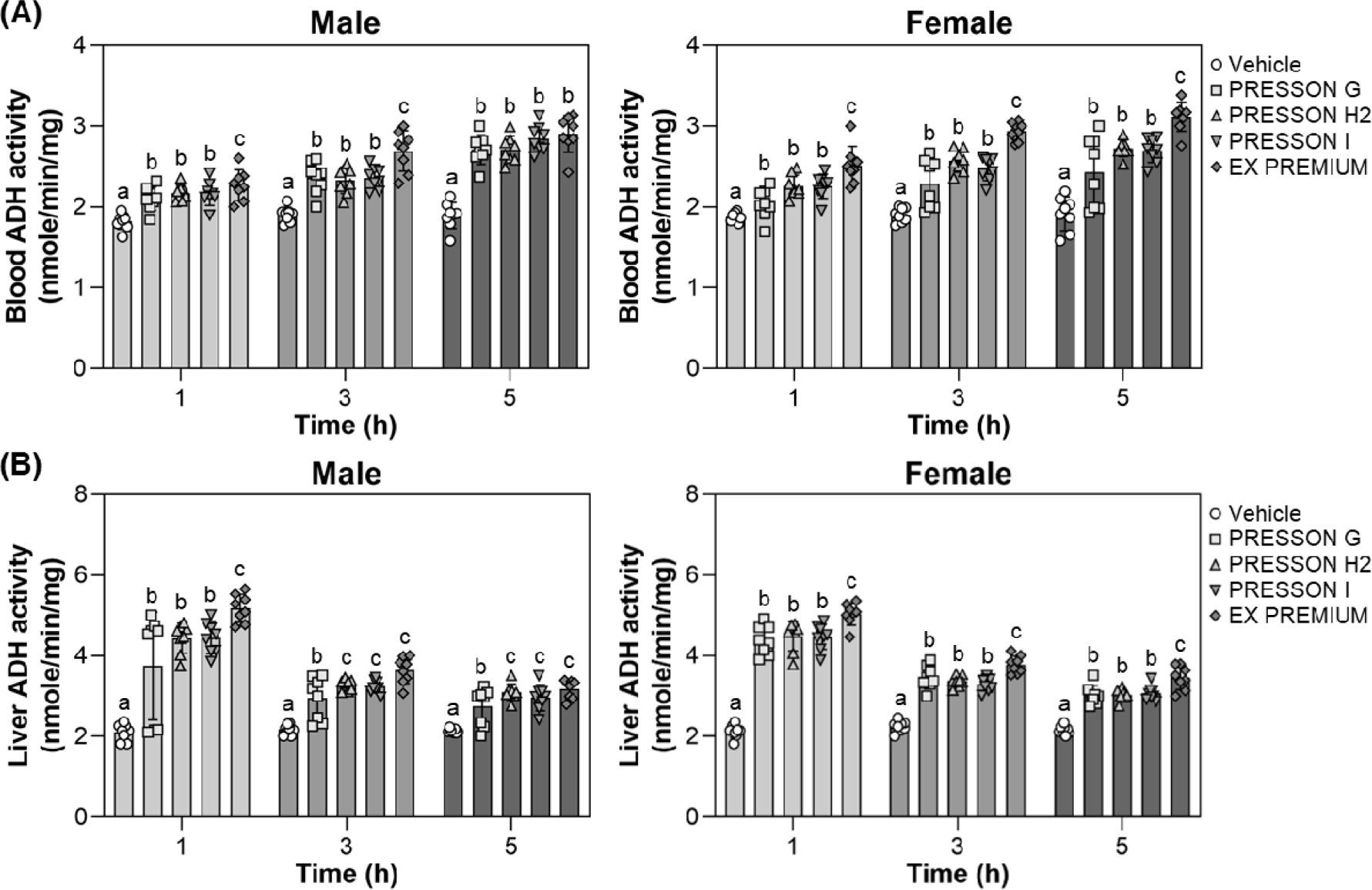
In the liver of male mice, ADH activity at 1 hour was significantly higher in all treatment conditions compared to vehicle, with EX PREMIUM showing the highest activity. At 3 and 5 hours, PRESSON H2, PRESSON I, and EX PREMIUM maintained significantly elevated ADH activity compared to vehicle, with no notable difference in PRESSON H2, PRESSON I, and EX PREMIUM (Fig. 2B). In the liver of female mice, ADH activity was also significantly increased at 1 hour in the EX PREMIUM group (5.03 nmole/min/mg). At 3 and 5 hours, EX PREMIUM maintained significantly higher ADH activity than vehicle (Fig. 2B), indicating a statistically significant enhancement of acetaldehyde clearance.
To assess the role of sample treatments in facilitating acetaldehyde clearance, ALDH activity was measured in the blood and liver of male and female mice at 1, 3, and 5 hours after ethanol feeding, which was administered 30 minutes following sample treatment. In male mice, blood ALDH activity at 1 hour was significantly increased by all treatments compared to vehicle, with EX PREMIUM and PRESSON I showing the highest activities. At 3 and 5 hours, ALDH activity remained significantly elevated in all treatment (Fig. 3A). In female mice, blood ALDH activity was significantly elevated by all treated conditions compared to vehicle at all time points. At 3 and 5 hours, EX PREMIUM showed the highest activity, EX PREMIUM exhibited the highest ALDH activity, significantly higher than vehicle and other treatments, suggesting enhanced enzymatic induction (Fig. 3A).
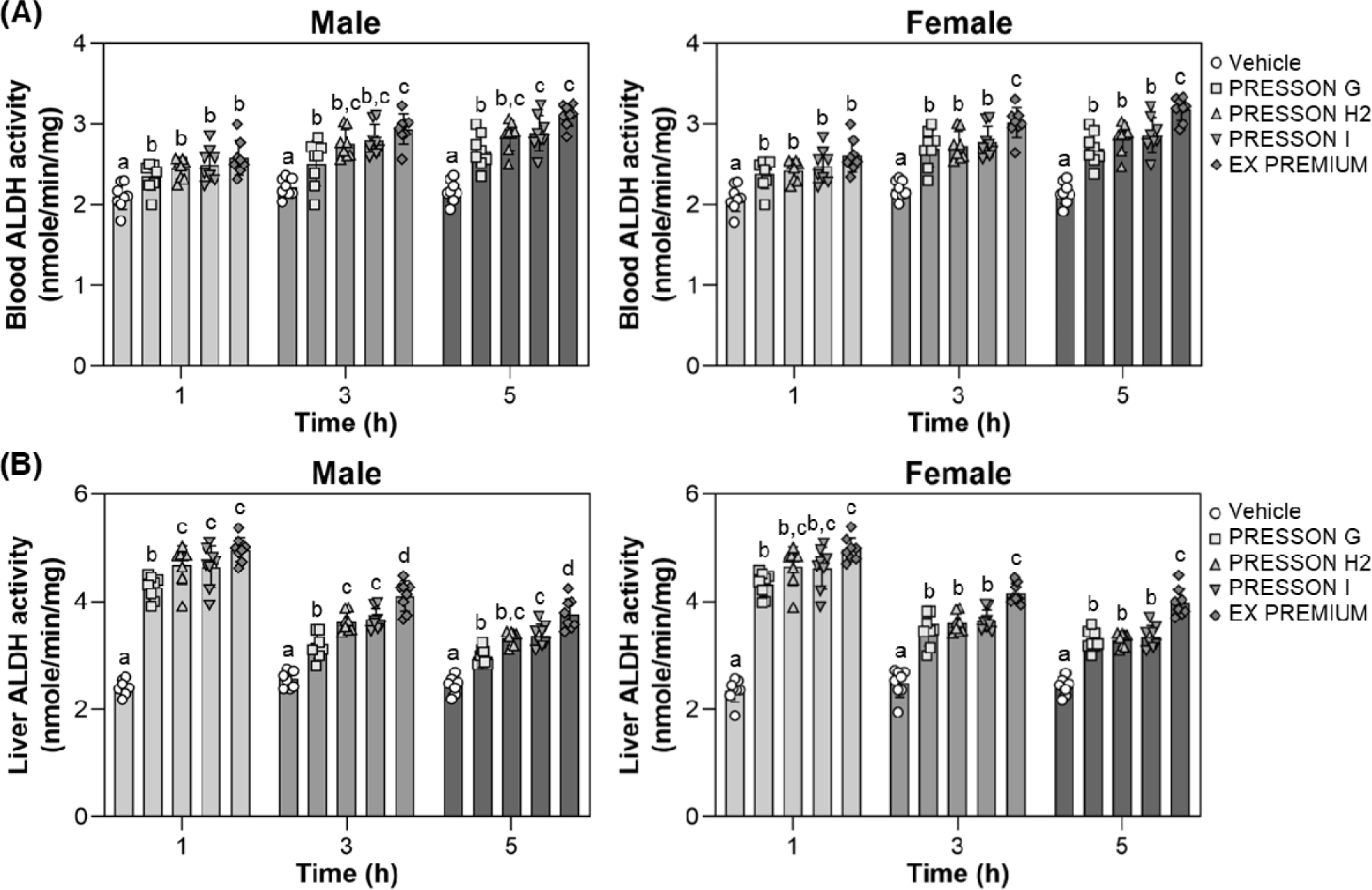
In the liver of male mice, ALDH activity was significantly increased by all Morning Care treatments at 1 hour, with EX PREMIUM, PRESSON I, and PRESSON H2 showing the highest levels. At 3 and 5 hours, EX PREMIUM and PRESSON I maintained significantly higher ALDH activity than vehicle, with EX PREMIUM showing the highest activity at 3 and 5 hours (Fig. 3B). In female mice, hepatic ALDH activity at 1 hour was significantly increased by all treatments, and PRESSON H2, PRESSON I, EX PREMIUM showed the greatest elevations. At 3 and 5 hours, EX PREMIUM consistently exhibited significantly higher ALDH activity compared to vehicle (Fig. 3B).
These results demonstrate that Morning Care products upregulated the activities of both ADH and ALDH in blood and liver after ethanol intake. EX PREMIUM, in particular, induced the most sustained and substantial increases in enzymatic activity, indicating its potential efficacy in enhancing ethanol metabolism and acetaldehyde clearance.
To explore the transcriptional response of alcohol-metabolizing enzymes, hepatic mRNA expression levels of ADH) and ALDH were measured at 1, 3, and 5 hours after ethanol administration. dksldkslIn male mice, ADH expression was not significantly altered at 1 hour. At 3 hours, EX PREMIUM showed a significant increase compared to vehicle, whereas other treatments did not result in statistically significant changes. At 5 hours, all Morning Care treatments significantly upregulated ADH expression relative to vehicle, with no significant difference among the treatment conditions (Fig. 4A). In female mice, ADH expression did not show a significant difference among groups at 1 or 3 hours. At 5 hours, expression was significantly increased in PRESSON I and EX PREMIUM compared to vehicle, while PRESSON G and H2 did not show a significant difference from the control (Fig. 4A).
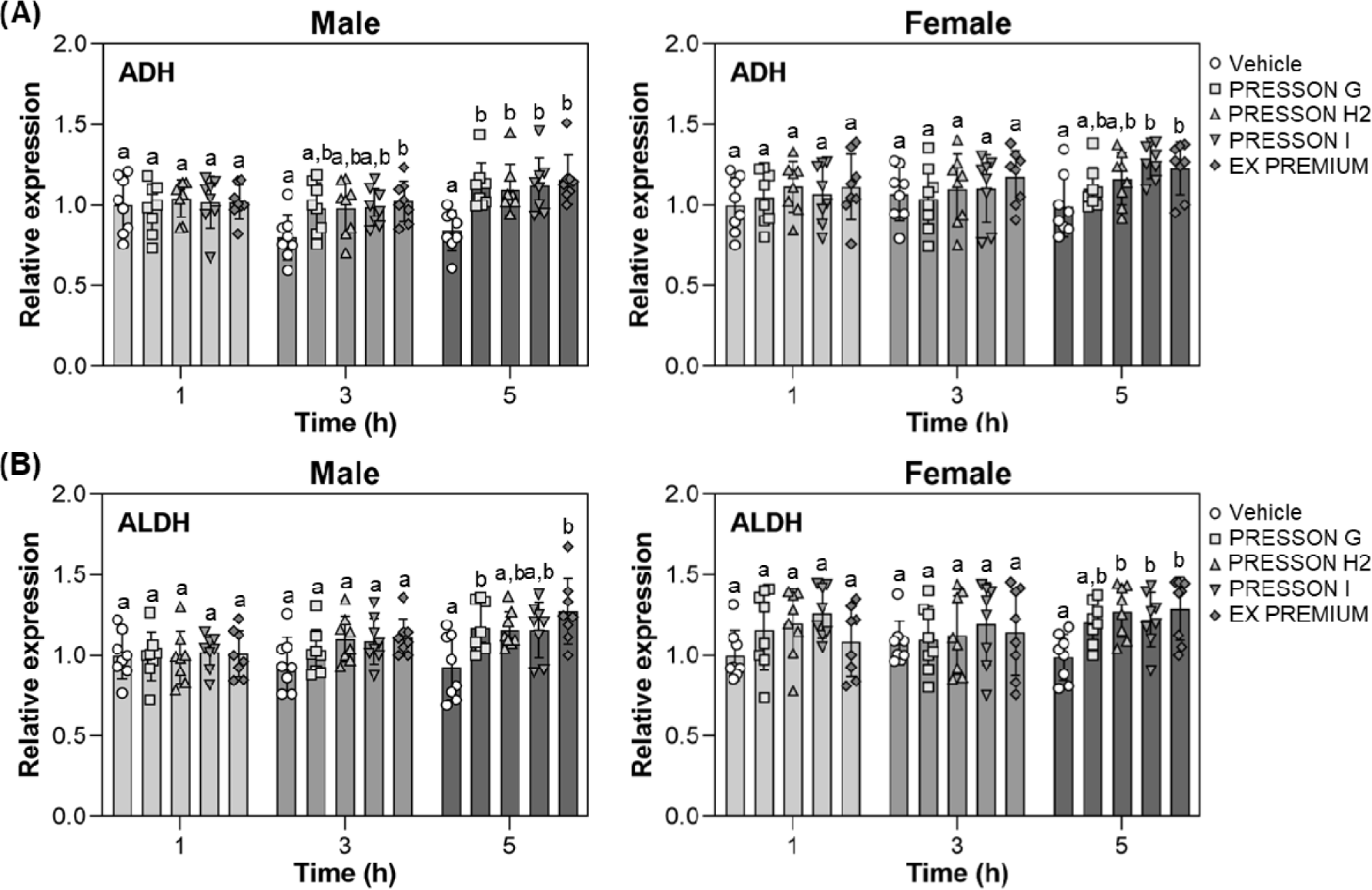
In male mice, ALDH expression did not differ significantly among groups at 1 or 3 hours. At 5 hours, expression levels were significantly elevated in PRESSON G and EX PREMIUM compared to vehicle, while PRESSON H2 and PRESSON I did not show significant changes (Fig. 4B). In female mice, ALDH expression at 5 hours was significantly elevated in PRESSON H2, PRESSON I, and EX PREMIUM compared to vehicle, whereas PRESSON G did not show a significant difference (Fig. 4B).
These results indicate that EX PREMIUM consistently upregulated hepatic ADH and ALDH gene expression, particularly at later time points, a potential role in enhancing the enzymatic capacity for ethanol and acetaldehyde metabolism.
Discussion
Efficient clearance of toxic metabolites is crucial for relieving alcohol-induced physiological discomfort. Notably, the accumulation of acetaldehyde, a major toxic metabolite of alcohol, is considered a key factor contributing to post-drinking discomfort. Several studies have demonstrated that enhancing the activity of alcohol-metabolizing enzymes such as ADH and ALDH, can promote the degradation of ethanol and acetaldehyde, thereby alleviating hangover symptoms and preventing alcohol-induced toxicity. Alcut, a natural extract mixture, not only enhanced the rapid initial degradation of ethanol but also sustained the activity of alcohol-metabolizing enzymes such as ADH and ALDH, thereby promoting continuous metabolism. Similarly, Cha et al. (2009) demonstrated that arginine and methionine simultaneously enhanced ADH and ALDH activity, facilitating the degradation of both ethanol and acetaldehyde. This dual effect was suggested to contribute not only to hangover relief but also to hepatic protection. Furthermore, a lysate obtained from Saccharomyces cerevisiae expressing ALDH significantly increased the activity of both ADH and ALDH enzymes and decreased blood acetaldehyde concentrations, indicating its potential in relieving hangover symptoms. These findings support the strategy of targeting both enzymatic (ADH and ALDH) activity and expression to improve alcohol metabolism. Notably, the activity and expression of these enzymes may also be influenced by sex-specific physiological factors. Meanwhile, Sex- based physiological differences also affect alcohol metabolism. Men typically exhibit more efficient first-pass hepatic metabolism than women, leading to lower blood alcohol concentrations and milder hangover symptoms under the same alcohol (Frezza et al., 1990; Seyedsadjadi et al., 2022). Based on these considerations, the present study included both male and female mice to evaluate sex-specific differences in metabolic responses and investigate the effects of the Morning Care series on alcohol metabolism.
The Morning Care series contain a range of functional ingredients, such as medicinal plant extracts, amino acids, and other bioactive compounds known to support alcohol metabolism and liver function. Among these, Hovenia dulcis fruit extract is perhaps the most well-documented in the context of hangover relief. It has been shown to enhance ADH and ALDH activities, facilitating the metabolism of ethanol and acetaldehyde, while also exhibiting hepatoprotective and antioxidant properties (He et al., 2024; Niiya et al., 2024). In addition to H. dulcis, several other bioactive components included in the Morning Care formulations may also contribute to hangover relief. Kudzu root extract (Pueraria lobata) has been reported to enhance ALDH2 activity, thereby accelerating acetaldehyde clearance (McGregor, 2007). Furthermore, Penetar et al. (2012) reported that isoflavones from kudzu plant reduce alcohol consumption and alter drinking behavior. Other studies have reported that persimmon fruit and leaf extracts enhance alcohol metabolism, reduce alcohol-induced liver damage, and improve lipid profiles through their antioxidant phenolic compounds (Moon & Cha, 2008; Wang et al., 2016). In addition to ginseng, particularly through its active components identified as ginsenosides, has been investigated for its hepatoprotective and antioxidative effects, showing beneficial roles in improving acute and chronic liver injuries, fatty liver, and hepatic fibrosis or cirrhosis (Huu Tung et al., 2012).
In the present study, Morning Care series significantly reduced blood concentrations of ethanol and acetaldehyde following ethanol administration. All Morning Care treatments led to a notable reduction in blood ethanol and acetaldehyde levels compared to the vehicle. EX PREMIUM demonstrated the most marked decrease in both metabolites, indicating the highest metabolic enhancement. This effect was closely associated with elevated ADH and ALDH enzyme activity. Enzyme activities in both blood and liver tissues were significantly increased in the sample-treated groups compared to the vehicle, with EX PREMIUM consistently showing the highest levels. Interestingly, hepatic enzyme activity peaked at 1 hour post-administration, while blood enzyme activity was highest at 5 hours. This time-dependent enzymatic response parallels the physiological course of alcohol metabolism, which begins with rapid hepatic degradation followed by sustained systemic clearance of residual ethanol and acetaldehyde. Additionally, ADH and ALDH gene expression was highest at 5 hours in both sexes, particularly in the EX PREMIUM group. These findings suggest that the increased enzyme activity involved not only post-translational regulation but also transcriptional upregulation.
In Korea, hangover-relief functionality statements in product labeling or advertising are permitted only when supported by objective and scientific human clinical data. To substantiate such indications, recognized evaluation indices include validated questionnaires assessing hangover symptoms, as well as measurements of blood ethanol and acetaldehyde concentrations. Comprehensive assessment of physiological and biochemical changes following alcohol consumption is required to verify hangover-relieving effects. In the present study, regulatory evaluation criteria were applied in a preclinical model. While preclinical, these findings may serve as foundational evidence supporting the hangover-relief potential of EX PREMIUM.
Taken together, EX PREMIUM demonstrated consistent and robust effects on both enzymatic activity and gene expression of alcohol-metabolizing enzymes, leading to enhanced clearance of ethanol and acetaldehyde. Such dual modulation at both enzymatic and transcriptional levels suggests a comprehensive enhancement of alcohol metabolism. These findings highlight its potential as a functional food or supplement for alleviating alcohol-related discomfort.
