Introduction
It’s essential to maintain an adequate intake of calcium from food or supplements to ensure optimal bodily functions and overall health. However, calcium is the first nutrient which intake is the lowest among Korean. Based on the 2020 Korea National Health and Nutrition Examination Survey (KDCA, 2021), the average calcium intake among adults in Korea was only 64.3% of the recommended amount, with 70% of the population failing to meet the adequate intake level. It was noted that calcium intake is particularly low among specific groups such as women, adolescents aged 12 to 18 years, and elderly people aged 65 years or older. To improve the calcium intake status in Korea, it is important to promote the consumption of dairy products and other calcium-rich foods, such as leafy greens, nuts, seeds, beans, tofu, sardines, and salmon, which is well known as natural Ca-rich food (Cormick & Belizán, 2019). And another good way is to develop calcium supplements to Ca deficient high-risk groups.
Volcanic seawater is naturally filtered and purified through volcanic rock layers, enriching it with a variety of minerals, including calcium (Ca), magnesium (Mg), iron (Fe), and other health-promoting elements (Noh et al., 2010; Mohd Nani et al., 2016). As a natural source of calcium, it can be utilized as a calcium supplement (Song et al., 2020). It has been reported that increasing calcium intake using calcium-rich mineral water can significantly enhance bone health (Vannucci et al., 2018). Some research indicates that drinking mineral water rich in calcium can substantially increase overall calcium intake (Pop et al., 2023), which is associated with reduced risk of osteoporosis. Jeju Island, known for its volcanic activity, has unique environmental conditions that influence the composition of its seawater. The seawater in this region is rich in minerals, especially Ca, due to the volcanic rocks and minerals that interact with the seawater. Therefore, calcium from Jeju Lava Seawater (CJLS) holds significance as a natural source of calcium.
However, despite the high calcium concentration in Jeju lava seawater, there has been no direct experience of consuming calcium extracted from lava seawater. Therefore, safety assessment of CJLS is essential to ensure safe consumption. To obtain calcium from CJLS, seawater is collected and then subjected to processes that concentrate and purify the calcium content. This process allows for the removal of unwanted elements, making it beneficial for individuals who need to limit specific minerals or substances. Verifying the toxicity of separated calcium is an essential procedure. In a previous study, we demonstrated the genetic stability and lack of mutagenicity of CJLS, indicating its non-toxicity and potential as a dependable and safe functional food ingredient (Kim & Park, 2023). In this study, we aim to conduct verification of acute and chronic toxicity which are essential process for clarifying the new ingredient. Acute toxicity refers to the harmful effects that occur from a single exposure to a substance within a short period, typically 24 hours. It is a measure of the severity of adverse effects caused by a new ingredient, usually evaluated in terms of the dose or concentration that leads to specific negative health outcomes including nausea, vomiting, diarrhea, respiratory distress, convulsions, or even death. Acute toxicity is commonly assessed using indicators such as the LD50 (lethal dose for 50% of a test population) or LC50 (lethal concentration for 50% of a test population), which represent the dose or concentration of a substance that results in the death of 50% of the subjects in a test group (CCOHS, 2024). When it comes to long-term toxicity, a 90-day oral toxicity study is a subchronic toxicity test where a substance is administered orally to test animals, usually rodents, daily for 90 days (OECD, 2018). Therefore, in this study, we aimed to conduct an acute toxicity test and a subchronic toxicity test to analyze the safety of CJLS. It helps in assessing the safety of chemicals, pharmaceuticals, and food additives by providing detailed information on the toxicological profile of the tested substance.
Materials and Methods
Jeju lave seawater flowed through underground aquifers, naturally filtered by underlying volcanic rock, in eastern Jeju. It was taken at a depth of 150 meters and filtered to remove foreign substances. JLS mineral extracts and desalinated water were obtained using a reverse osmosis system (SWRO, Woosung Co. Ltd, Eumseong, Korea) operated at a pressure of 1.8 bar or higher. Jeju lave seawater mineral extract contains calcium, magnesium, potassium, sodium and trace minerals. The CJLS was made by evaporating (45–70°C), washing and air drying (over 90°C) the JLS mineral extracts using the difference in solubility. CJLS contains of calcium (26.36±0.53%), magnesium (0.12±0.004%) and moisture (under 10%).
In this study, the study followed the GLP regulations (Taegu Haany University, Korea) to investigate toxicity regarding acute (IACUC-2022-033-2), subacute (IACUC-2022-032-2), and subchronic toxicity test (IACUC-2022-047-2). The acute oral toxicity of CJLS was assessed in the mice in accordance with the method of the Organization for Economic Cooperation and Development (OECD) Guideline No. 423 (OECD, 2002). The subacute toxicity study was conducted in accordance with and adhered to the protocol outlined in OECD Guideline No. 407 (OECD, 2008). The 90-day repeated-dose oral toxicity study was conducted following OECD guidelines No. 408 (OECD, 2018).
A single-dose toxicity study was conducted to evaluate the toxicity of CJLS in 8-week-old Sprague-Dawley rats (146.8–228.5 g) following a single oral administration. The experimental groups were randomized to ensure each group had an equal average weight, with two male and two female groups, each containing five animals. The animals were fasted for approximately 15–18 hours on the day before administration of the CJLS, which was given orally once per day at doses of 0 or 2,000 mg/kg. The control group was administered with sterile saline solution (calcium concentration 0 mg/kg). On the day of administration, observations were made at 30 minutes, 1–, 2–, 4–, and 6-hours post-administration, and general symptoms were monitored once daily until necropsy. Mortality, general symptoms, and body weight change of all animals were measured on day 14 after administration. The animals were euthanized by exsanguination via the abdominal aorta and vein under isoflurane anesthesia, followed by necropsy. Necropsy was performed to conduct a macroscopic examination of the organs.
A total of 40 Sprague–Dawley (SD) rats, 6 weeks old (weighing between 122.8 and 187.6 g, with 20 males and 20 females), were utilized for the 28-day repeated-dose oral toxicity study. The animals were housed individually in plastic cages, separated by sex, and sustained under controlled conditions: temperature, 22±3°C; relative humidity, 30–70%; and a 12 h light/dark cycle. The rats were provided with a Teklad Certified Irradiated Global 18% protein rodent diet (ENVIGO, Life Sciences, Indianapolis, IN, USA) and ad libitum water access. Forty SD rats were randomly assigned to one control group (G1) and three treatment groups (G2–G4, n=10; 5 males and 5 females per group). CJLS was administered once daily via oral gavage for 28 sequential days at doses of 250, 500, and 1,000 mg/kg/day, respectively. The control group received only physiological saline (normal saline). Throughout the 28-day subacute toxicity trial, all experimental animals were observed twice daily for general symptoms, changes in body weight, mortality, and any signs of gross toxicity. At the end of the drug administration period (over 17 hours after the final dose), all surviving rats in each group were euthanized for analysis of hematological and biochemical indicators, histopathology, and relative organ weight (ROW) (Han et al., 2019; Wang et al., 2020).
For repeated-dose oral toxicity of 90 days, total of 80 SD rats, age 6 weeks (130.1–186.9 g, 20 males and 20 females) were used. The animals were housed individually in plastic cages, separated by sex, and sustained under controlled conditions: temperature, 22±3°C; relative humidity, 30–70%; and a 12 h light/dark cycle. The rats were administrated with a Teklad Certified Irradiated Global 18% protein rodent diet (ENVIGO, Life sciences, Indianapolis, IN, USA) and had free access to water. The SD rats were randomly divided into a control group (G1) and three treatment groups (G2–G4, n=20; 10 males and 10 females per group). CJLS was administered once daily via oral gavage for 90 sequential days at doses of 250, 500, and 1,000 mg/kg/day, respectively. The control group received only normal saline. All the experimental animals were monitored twice daily for general symptoms, including weight changes, functional observations, and mortality, throughout the 90-day toxicity trial. At the end of the CJLS administration period (over 17 hours after the final dose), all surviving rats in each group were euthanized for analysis of hematological and biochemical indicators, histopathology, and ROW.
Clinical observations were recorded once a day to monitor signs of toxicity, particularly at a consistent time each day (1 hour after CJLS or vehicle administration). Each rat’s body weight was measured prior to CJLS administration on Day 1, then daily throughout the experimental period, and again on the day of necropsy. The necropsy body weight was recorded following a 17-hour fast.
Upon completion of the trial, all the surviving rats were deprived of food for 17 hours (with water permitted) before blood sampling. Blood was collected into EDTA-containing tubes for hematology and biochemistry parameter analyses, respectively. Blood profiles such as red blood cell count (RBC), white blood cell count (WBC), hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red dell distribution width (RDW), hemoglobin distribution width (HDW), mean platelet volume (MPV), platelet count (PLT), neutrophils (NEU) %, lymphocyte (LYM) %, monocyte (MONO) %, eosinophis (EOS) %, large unstrained cells (LUC) %, and basophils (BASO) %, were measured using an animal blood cell analyzer (ADVIA 2120, SIEMES, Erlangen, Bavaria, Germany) by establishing the biological reference intervals specific to the sex and strain of the rats.
Blood samples for serum biochemical assays were allowed to clot for 2 hours, followed by centrifugation at 3,000 rpm and 4°C for 10 minutes, and subsequently stored at −20°C until further analysis. The main parameters included were albumin (ALB), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), total bilirubin (BIL), blood urea nitrogen (BUN), Ca, total cholesterol (CHO), creatinine (CRE), gamma- glutamyl transpeptidase (GGT), glucose (GLU), inorganic phosphorus (IP), total protein (PRO), triglyceride (TG), albumin/globulin ratio (A/G ratio), Na+, K+, and Cl– were measured with an automatic serum biochemistry analyzer (KONELAB 20XT, Thermo, MA, USA) by establishing the biological reference intervals specific to the sex and strain of the rats. Electrocyte was analyzed by electrolyte analyzer (i-Smakrt30, i-sense, Seoul, Korea).
Data were provided as mean±standard deviation (SD). The mean differences between the control and CJLS groups were considered using one-way analysis of variance (ANOVA), followed by and Dunnett’s Tukey’s post-hoc test for the 28-day and 90-day repeated-dose toxicity studies (SPSS Statistics 19.0, IBM, Chicago, IL, USA).
Results and Discussion
Our results indicate that the administration of CJLS did not induce any significant modifications in the mean body weight of rats during the acute, 28-day, and 90-day administration periods (Fig. 1). This lack of significant body weight changes across different dosages have demonstrated in the absence of toxic effects from natural mineral supplements.
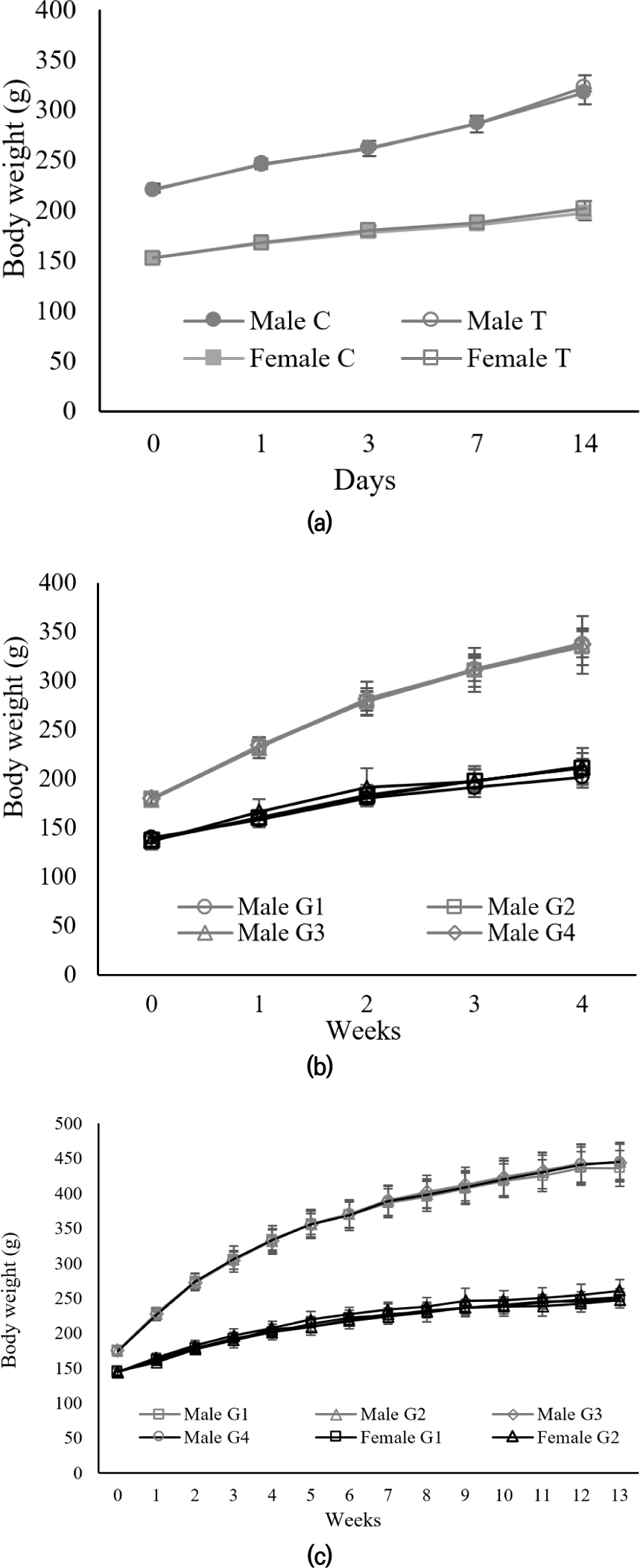
A reduction in body weight is considered a sensitive and straightforward indicator of toxicity, with a 5% decrease in body weight widely accepted as predictive of pathological outcomes (Silva et al., 2021). However, in this study, throughout the 90-day experiment, there was no significant difference observed in body weight change between the treated group rats and the control group.
Throughout the 14-day observation period following a single oral administration, no mortality or behavioral abnormalities (such as lethargy, sleep disturbances, coma, tremors, or diarrhea) were observed in any of the mice. Furthermore, no significant pathological changes were observed in the color or texture of vital organs, including the heart, lungs, liver, spleen, thymus, kidneys, ovaries or testes, and gastrointestinal tract. Therefore, under these experimental conditions, the approximate lethal dose of CJLS in SD rats is estimated to exceed 2,000 mg/kg for both males and females.
Following 28 consecutive days of administration, no statistically significant differences were observed in the ROWs among all groups at any dosage level. All minor variations in ROW values remained within the normal range (data not shown).
In toxicology studies, organ weight and tissue weight ratios are important because they provide critical insights into the effects of toxic substances on the body’s organs and tissues. Changes in organ weight can indicate toxicity targeted at specific organs. For example, an increase or decrease in liver weight may suggest hepatotoxicity due to exposure to certain chemicals. By examining the ratio of organ weight to body weight or to another organ’s weight, researchers can establish dose-response relationships. This helps determine if the toxic effects increase proportionally with the dose of the substance administered. Alterations in organ weight ratios often correlate with functional impairments. Alterations in heart weight relative to body weight may indicate cardiac dysfunction induced by toxicants that impact cardiovascular health (Bailey et al., 2004; Michael et al., 2007; Amresh et al., 2008).
In this study, there were no significant weight differences observed among the organs, indicating that the substance is free from all toxic effects. However, significant changes were partially observed in the absolute weights of the right adrenal gland, thymus, and spleen in male rats, and the lungs in female rats, compared to the control group. However, these changes were not dose-dependent, suggesting that they are not attributable to the test substance.
As revealed in Table 1, all the measured blood parameters had no remarkable differences among the three CJLS-treated groups and the control group for oral 28-days administration test.
Subacute oral administration of CJLS for 28 days did not affect significant variations in most of the biochemical parameters, comprising the level of liver enzymes (AST and ALT), serum ALP, BUN, CRE, TBL, TC, TG, ALB, TP in male and female rats (Table 2). Typically, elevated levels of AST and ALT are commonly linked to liver damage or stress, as these enzymes are released into the bloodstream when liver cells are damaged (Pratt et al., 2000; Giannini et al., 2005; Ozer et al., 2008). The stability of these markers in the treated groups suggests that CJLS does not exert adverse effects on liver function, which is a positive indicator of its safety profile. Similarly, BUN and CRE serve as essential indicators of renal function. Increased levels of these markers typically indicate impaired kidney function or damage, as the kidneys are responsible for filtering and excreting waste products from the blood (Stevens et al., 2006; Rahman et al., 2012). The lack of significant changes in BUN and CRE levels further supports the conclusion that CJLS does not adversely affect renal function at the doses tested. Additionally, the absence of significant changes in serum TC, TG, PRO, and ALB indicates that CJLS does not disrupt lipid metabolism or protein synthesis (Rifai et al., 1992; Doumas & Peter 1997; Wu 2016). This is particularly important as alterations in these parameters can signal metabolic dysfunction, nutritional deficiencies, or systemic inflammation. the biochemical data from this study provide strong evidence that CJLS, when administered subacutely for 28 days, does not induce significant toxicological effects on liver, kidney, or metabolic functions in rats. This supports the potential for safe long-term use of CJLS as a dietary supplement.
Ninety days of oral administration of the CLJS did not cause any behavioral changes in the treated groups. No death or adverse effects were noted throughout the experiment. In male rats, heart weight significantly decreased in all test groups (G2–G4) compared to the control group (p<0.05). However, the decrease was minimal and showed no dose dependency, indicating that the changes were not due to the test substance. In female rats, thymus weight significantly decreased in the low-dose group (G2) compared to the control group, but the decrease was minimal and showed no dose dependency, suggesting that the changes were not due to the test substance (Fig. 2). The data did not demonstrate any clear dose- response relationships, and all minor fluctuations in the following parameters for both sexes were within the normal range of the testing laboratory.
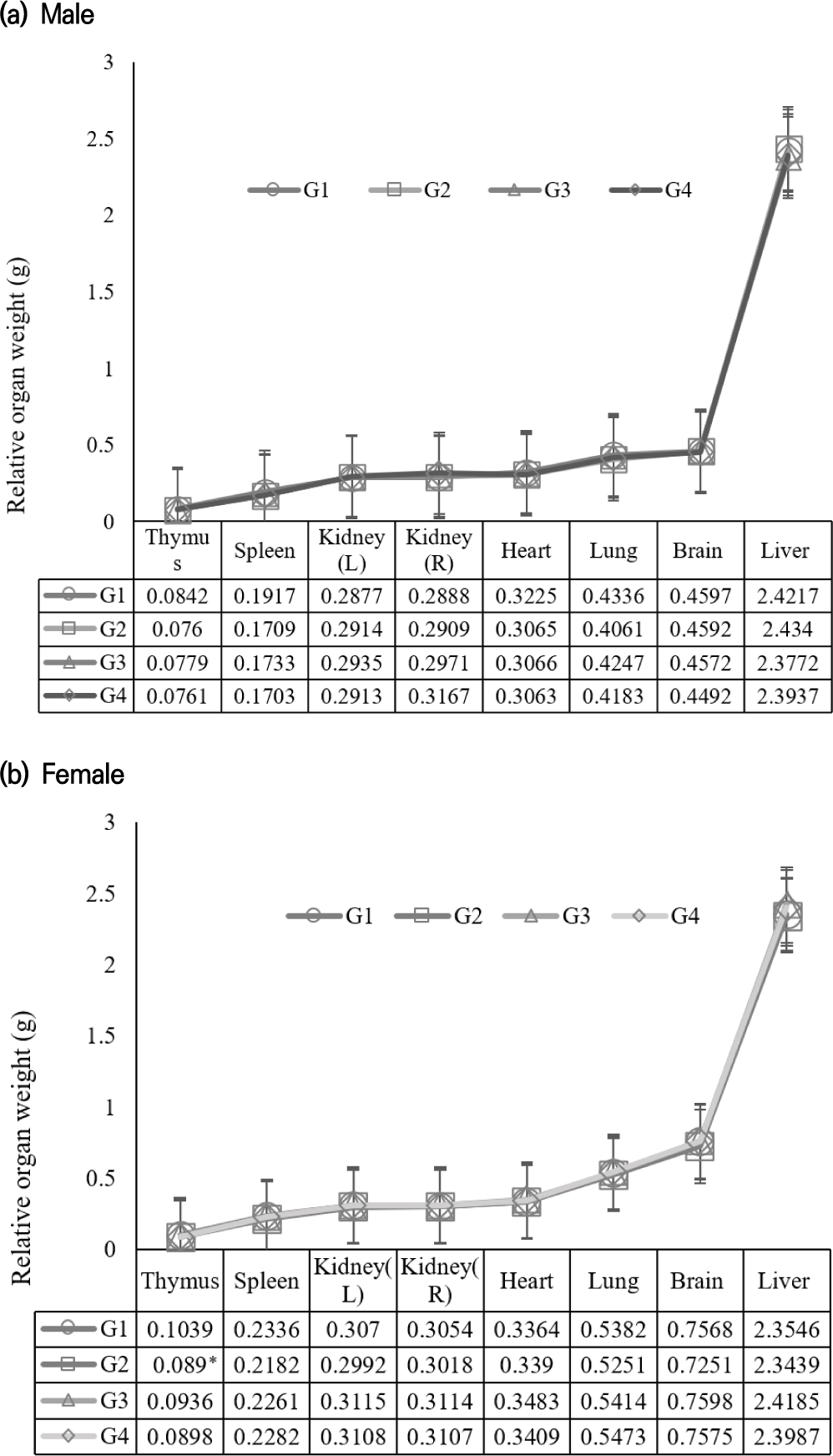
No abnormality was observed in the hematological parameters after 90 days of CJLS administration (Table 3). In female rats, the RBC count significantly increased in the high-dose group (G4) compared to the control group; however, the increase was minimal and lacked dose dependency.
The 90-day oral administration of CJLS did not result in significant changes in most biochemical parameters. These parameters include serum ALP, TBL, BUN, CRE, TC, TG, TP, ALB, as well as the liver enzymes AST and ALT, in both male and female rats (Table 4). In male rat, T3 level significantly decreased in the low-dose group (G2) compared to the control group, but the decrease was minimal and had no dose dependency.
In addition, no abnormalities were detected in the gross examination of organs between the CJLS groups and control groups. No inflammation, internal bleeding, lesions, or deformities were observed in the liver, kidneys, testes, spleen, and heart of treated rats compared to the control group after 90 days of CJLS extract administration at various doses. The histological sections of the heart, liver, kidneys, testes, and spleen showed no abnormalities in the architectural structure of the organs in any group. Calcium plays a very crucial role in human physiological functions, and natural calcium supplements can aid human health. Jeju lava seawater is thought to have the potential to serve as a natural calcium supplement. This study was conducted to assess the acute and subchronic toxicity of CJLS. Based on the conducted study, no CJLS-related deaths and no additional clinical abnormalities were detected during the acute and subacute experimental periods. The blood hematology and serum biochemistry data showed no differences between the CJLS groups and control groups, and histopathological examination revealed no toxic effects in the vital or reproductive organs at any dose level. Therefore, the oral administration of CJLS at a dose lower than 1,000 mg/kg BW in one day is safe for the rats, which provided a basis for the functional use of CJLS and for determining a reasonable safe dose.
